ROS-mediated lysosomal membrane permeabilization and autophagy inhibition regulate bleomycin-induced cellular senescence
- PMID: 38762757
- PMCID: PMC11346523
- DOI: 10.1080/15548627.2024.2353548
ROS-mediated lysosomal membrane permeabilization and autophagy inhibition regulate bleomycin-induced cellular senescence
Abstract
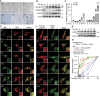
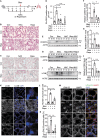
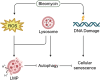
Bleomycin exhibits effective chemotherapeutic activity against multiple types of tumors, and also induces various side effects, such as pulmonary fibrosis and neuronal defects, which limit the clinical application of this drug. Macroautophagy/autophagy has been recently reported to be involved in the functions of bleomycin, and yet the mechanisms of their crosstalk remain insufficiently understood. Here, we demonstrated that reactive oxygen species (ROS) produced during bleomycin activation hampered autophagy flux by inducing lysosomal membrane permeabilization (LMP) and obstructing lysosomal degradation. Exhaustion of ROS with N-acetylcysteine relieved LMP and autophagy defects. Notably, we observed that LMP and autophagy blockage preceded the emergence of cellular senescence during bleomycin treatment. In addition, promoting or inhibiting autophagy-lysosome degradation alleviated or exacerbated the phenotypes of senescence, respectively. This suggests the alternation of autophagy activity is more a regulatory mechanism than a consequence of bleomycin-induced cellular senescence. Taken together, we reveal a specific role of bleomycin-induced ROS in mediating defects of autophagic degradation and further regulating cellular senescence in vitro and in vivo. Our findings, conversely, indicate the autophagy-lysosome degradation pathway as a target for modulating the functions of bleomycin. These provide a new perspective for optimizing bleomycin as a clinically applicable chemotherapeutics devoid of severe side-effects.Abbreviations: AT2 cells: type II alveolar epithelial cells; ATG7: autophagy related 7; bEnd.3: mouse brain microvascular endothelial cells; BNIP3L: BCL2/adenovirus E1B interacting protein 3-like; CCL2: C-C motif chemokine ligand 2; CDKN1A: cyclin dependent kinase inhibitor 1A; CDKN2A: cyclin dependent kinase inhibitor 2A; FTH1: ferritin heavy polypeptide 1; γ-H2AX: phosphorylated H2A.X variant histone; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; HUVEC: human umbilical vein endothelial cells; HT22: hippocampal neuronal cell lines; Il: interleukin; LAMP: lysosomal-associated membrane protein; LMP: lysosome membrane permeabilization; MTORC1: mechanistic target of rapamycin kinase complex 1; NAC: N-acetylcysteine; NCOA4: nuclear receptor coactivator 4; PI3K: phosphoinositide 3-kinase; ROS: reactive oxygen species; RPS6KB/S6K: ribosomal protein S6 kinase; SA-GLB1/β-gal: senescence-associated galactosidase, beta 1; SAHF: senescence-associated heterochromatic foci; SASP: senescence-associated secretory phenotype; SEC62: SEC62 homolog, preprotein translocation; SEP: superecliptic pHluorin; SQSTM1/p62: sequestosome 1; TFEB: transcription factor EB.
Keywords: Autophagy; ROS; bleomycin; cellular senescence; lysosomal membrane permeabilization.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
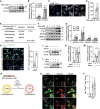
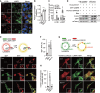
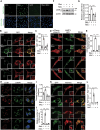
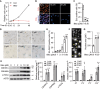

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
