Bunyavirus SFTSV NSs utilizes autophagy to escape the antiviral innate immune response
- PMID: 38762760
- PMCID: PMC11423686
- DOI: 10.1080/15548627.2024.2356505
Bunyavirus SFTSV NSs utilizes autophagy to escape the antiviral innate immune response
Abstract
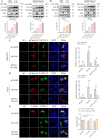
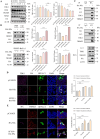
Severe fever with thrombocytopenia syndrome virus (SFTSV) nonstructural protein (NSs) is an important viral virulence factor that sequesters multiple antiviral proteins into inclusion bodies to escape the antiviral innate immune response. However, the mechanism of the NSs restricting host innate immunity remains largely elusive. Here, we found that the NSs induced complete macroautophagy/autophagy by interacting with the CCD domain of BECN1, thereby promoting the formation of a BECN1-dependent autophagy initiation complex. Importantly, our data showed that the NSs sequestered antiviral proteins such as TBK1 into autophagic vesicles, and therefore promoted the degradation of TBK1 and other antiviral proteins. In addition, the 8A mutant of NSs reduced the induction of BECN1-dependent autophagy flux and degradation of antiviral immune proteins. In conclusion, our results indicated that SFTSV NSs sequesters antiviral proteins into autophagic vesicles for degradation and to escape antiviral immune responses.
Keywords: Autophagy; SFTSV; TBK1; immune escape; inclusion bodies.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





Similar articles
-
SFTSV NSs degrades SAFA via autophagy to suppress SAFA-dependent antiviral response.PLoS Pathog. 2025 Jun 3;21(6):e1013201. doi: 10.1371/journal.ppat.1013201. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40460159 Free PMC article.
-
Two Conserved Amino Acids within the NSs of Severe Fever with Thrombocytopenia Syndrome Phlebovirus Are Essential for Anti-interferon Activity.J Virol. 2018 Sep 12;92(19):e00706-18. doi: 10.1128/JVI.00706-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021900 Free PMC article.
-
Nonstructural Protein of Severe Fever with Thrombocytopenia Syndrome Phlebovirus Inhibits TBK1 to Evade Interferon-Mediated Response.J Microbiol Biotechnol. 2021 Feb 28;31(2):226-232. doi: 10.4014/jmb.2008.08048. J Microbiol Biotechnol. 2021. PMID: 33397830 Free PMC article.
-
Unraveling the Underlying Interaction Mechanism Between Dabie bandavirus and Innate Immune Response.Front Immunol. 2021 May 27;12:676861. doi: 10.3389/fimmu.2021.676861. eCollection 2021. Front Immunol. 2021. PMID: 34122440 Free PMC article. Review.
-
The Role of Non-Structural Protein NSs in the Pathogenesis of Severe Fever with Thrombocytopenia Syndrome.Viruses. 2021 May 11;13(5):876. doi: 10.3390/v13050876. Viruses. 2021. PMID: 34064604 Free PMC article. Review.
Cited by
-
SFTSV NSs degrades SAFA via autophagy to suppress SAFA-dependent antiviral response.PLoS Pathog. 2025 Jun 3;21(6):e1013201. doi: 10.1371/journal.ppat.1013201. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40460159 Free PMC article.
-
Growth Arrest and DNA Damage Protein 45A Promotes PPRV Replication via the Downregulation of TBK1 Expression to Inhibit IFN-β Signaling Pathway.FASEB J. 2025 Jun 30;39(12):e70741. doi: 10.1096/fj.202500364R. FASEB J. 2025. PMID: 40548900 Free PMC article.
-
Bunyavirus SFTSV nucleoprotein exploits TUFM-mediated mitophagy to impair antiviral innate immunity.Autophagy. 2025 Jan;21(1):102-119. doi: 10.1080/15548627.2024.2393067. Epub 2024 Sep 12. Autophagy. 2025. PMID: 39189526 Free PMC article.
-
Latest advances and prospects in the pathogenesis, animal models, and vaccine research of severe fever with thrombocytopenia syndrome virus.Front Immunol. 2025 Jun 26;16:1624290. doi: 10.3389/fimmu.2025.1624290. eCollection 2025. Front Immunol. 2025. PMID: 40642074 Free PMC article. Review.
-
Current insights into human pathogenic phenuiviruses and the host immune system.Virulence. 2024 Dec;15(1):2384563. doi: 10.1080/21505594.2024.2384563. Epub 2024 Jul 29. Virulence. 2024. PMID: 39072499 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
