Acetaminophen for Prevention and Treatment of Organ Dysfunction in Critically Ill Patients With Sepsis: The ASTER Randomized Clinical Trial
- PMID: 38762798
- PMCID: PMC11304120
- DOI: 10.1001/jama.2024.8772
Acetaminophen for Prevention and Treatment of Organ Dysfunction in Critically Ill Patients With Sepsis: The ASTER Randomized Clinical Trial
Abstract
Importance: Acetaminophen (paracetamol) has many pharmacological effects that might be beneficial in sepsis, including inhibition of cell-free hemoglobin-induced oxidation of lipids and other substrates.
Objective: To determine whether acetaminophen increases days alive and free of organ dysfunction in sepsis compared with placebo.
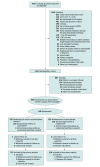
Design, setting, and participants: Phase 2b randomized, double-blind, clinical trial conducted from October 2021 to April 2023 with 90-day follow-up. Adults with sepsis and respiratory or circulatory organ dysfunction were enrolled in the emergency department or intensive care unit of 40 US academic hospitals within 36 hours of presentation.
Intervention: Patients were randomized to 1 g of acetaminophen intravenously every 6 hours or placebo for 5 days.
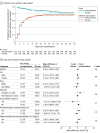
Main outcome and measures: The primary end point was days alive and free of organ support (mechanical ventilation, vasopressors, and kidney replacement therapy) to day 28. Treatment effect modification was evaluated for acetaminophen by prerandomization plasma cell-free hemoglobin level higher than 10 mg/dL.
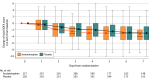
Results: Of 447 patients enrolled (mean age, 64 [SD, 15] years, 51% female, mean Sequential Organ Failure Assessment [SOFA] score, 5.4 [SD, 2.5]), 227 were randomized to acetaminophen and 220 to placebo. Acetaminophen was safe with no difference in liver enzymes, hypotension, or fluid balance between treatment arms. Days alive and free of organ support to day 28 were not meaningfully different for acetaminophen (20.2 days; 95% CI, 18.8 to 21.6) vs placebo (19.6 days; 95% CI, 18.2 to 21.0; P = .56; difference, 0.6; 95% CI, -1.4 to 2.6). Among 15 secondary outcomes, total, respiratory, and coagulation SOFA scores were significantly lower on days 2 through 4 in the acetaminophen arm as was the rate of development of acute respiratory distress syndrome within 7 days (2.2% vs 8.5% acetaminophen vs placebo; P = .01; difference, -6.3; 95% CI, -10.8 to -1.8). There was no significant interaction between cell-free hemoglobin levels and acetaminophen.
Conclusions and relevance: Intravenous acetaminophen was safe but did not significantly improve days alive and free of organ support in critically ill sepsis patients.
Trial registration: ClinicalTrials.gov Identifier: NCT04291508.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL123018/HL/NHLBI NIH HHS/United States
- U01 HL123023/HL/NHLBI NIH HHS/United States
- U01 HL122989/HL/NHLBI NIH HHS/United States
- U01 HL123008/HL/NHLBI NIH HHS/United States
- U01 HL123027/HL/NHLBI NIH HHS/United States
- U01 HL123033/HL/NHLBI NIH HHS/United States
- U01 HL123022/HL/NHLBI NIH HHS/United States
- U01 HL123010/HL/NHLBI NIH HHS/United States
- U01 HL123004/HL/NHLBI NIH HHS/United States
- U01 HL123031/HL/NHLBI NIH HHS/United States
- U01 HL123020/HL/NHLBI NIH HHS/United States
- U01 HL123009/HL/NHLBI NIH HHS/United States
- U01 HL122998/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

