Analysis of meiotic recombination in Drosophila simulans shows no evidence of an interchromosomal effect
- PMID: 38762892
- PMCID: PMC11304986
- DOI: 10.1093/genetics/iyae084
Analysis of meiotic recombination in Drosophila simulans shows no evidence of an interchromosomal effect
Abstract
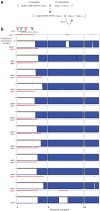
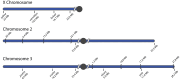
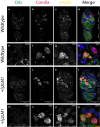
Chromosome inversions are of unique importance in the evolution of genomes and species because when heterozygous with a standard arrangement chromosome, they suppress meiotic crossovers within the inversion. In Drosophila species, heterozygous inversions also cause the interchromosomal effect, whereby the presence of a heterozygous inversion induces a dramatic increase in crossover frequencies in the remainder of the genome within a single meiosis. To date, the interchromosomal effect has been studied exclusively in species that also have high frequencies of inversions in wild populations. We took advantage of a recently developed approach for generating inversions in Drosophila simulans, a species that does not have inversions in wild populations, to ask if there is an interchromosomal effect. We used the existing chromosome 3R balancer and generated a new chromosome 2L balancer to assay for the interchromosomal effect genetically and cytologically. We found no evidence of an interchromosomal effect in D. simulans. To gain insights into the underlying mechanistic reasons, we qualitatively analyzed the relationship between meiotic double-stranded break (DSB) formation and synaptonemal complex (SC) assembly. We found that the SC is assembled prior to DSB formation as in D. melanogaster; however, we show that the SC is assembled prior to localization of the oocyte determination factor Orb, whereas in D. melanogaster, SC formation does not begin until the Orb is localized. Together, our data show no evidence that heterozygous inversions in D. simulans induce an interchromosomal effect and that there are differences in the developmental programming of the early stages of meiosis.
Keywords: Drosophila simulans; inversion; meiosis.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest The author(s) declare no conflict of interest.
Figures

Similar articles
-
Local Inversion Heterozygosity Alters Recombination throughout the Genome.Curr Biol. 2018 Sep 24;28(18):2984-2990.e3. doi: 10.1016/j.cub.2018.07.004. Epub 2018 Aug 30. Curr Biol. 2018. PMID: 30174188 Free PMC article.
-
Effects of autosomal inversions on meiotic exchange in distal and proximal regions of the X chromosome in a natural population of Drosophila melanogaster.Genet Res. 1994 Feb;63(1):57-62. doi: 10.1017/s0016672300032080. Genet Res. 1994. PMID: 8206367
-
The occurrence of double strand DNA breaks is not the sole condition for meiotic crossing over in Drosophila melanogaster.Genetica. 2000;108(1):87-90. doi: 10.1023/a:1004003319569. Genetica. 2000. PMID: 11145425
-
Female Meiosis: Synapsis, Recombination, and Segregation in Drosophila melanogaster.Genetics. 2018 Mar;208(3):875-908. doi: 10.1534/genetics.117.300081. Genetics. 2018. PMID: 29487146 Free PMC article. Review.
-
Meiotic recombination and chromosome segregation in Drosophila females.Annu Rev Genet. 2002;36:205-32. doi: 10.1146/annurev.genet.36.041102.113929. Epub 2002 Jun 11. Annu Rev Genet. 2002. PMID: 12429692 Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

