Leveraging neural plasticity for the treatment of amblyopia
- PMID: 38763223
- PMCID: PMC11380599
- DOI: 10.1016/j.survophthal.2024.04.006
Leveraging neural plasticity for the treatment of amblyopia
Abstract
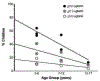
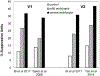
Amblyopia is a form of visual cortical impairment that arises from abnormal visual experience early in life. Most often, amblyopia is a unilateral visual impairment that can develop as a result of strabismus, anisometropia, or a combination of these conditions that result in discordant binocular experience. Characterized by reduced visual acuity and impaired binocular function, amblyopia places a substantial burden on the developing child. Although frontline treatment with glasses and patching can improve visual acuity, residual amblyopia remains for most children. Newer binocular-based therapies can elicit rapid recovery of visual acuity and may also improve stereoacuity in some children. Nevertheless, for both treatment modalities full recovery is elusive, recurrence of amblyopia is common, and improvements are negligible when treatment is administered at older ages. Insights derived from animal models about the factors that govern neural plasticity have been leveraged to develop innovative treatments for amblyopia. These novel therapies exhibit efficacy to promote recovery, and some are effective even at ages when conventional treatments fail to yield benefit. Approaches for enhancing visual system plasticity and promoting recovery from amblyopia include altering the balance between excitatory and inhibitory mechanisms, reversing the accumulation of proteins that inhibit plasticity, and harnessing the principles of metaplasticity. Although these therapies have exhibited promising results in animal models, their safety and ability to remediate amblyopia need to be evaluated in humans.
Keywords: Amblyopia; Critical period; Excitatory/inhibitory balance; Metaplasticity; Neuroplasticity; Suppression.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

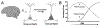
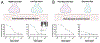
References
Key articles
-
- Birch EE, Kelly KR. Amblyopia and the whole child. Prog Ret Eye Res. 2023;93:101168. - PMC - PubMed
-
A recent review that highlights the complex sensory and ocular motor presentation of amblyopia, factors that may influence individual variability in treatment response, and the broad impact of amblyopia on the whole child.
-
- Cruz OA, Repka MX, Hercinovic A, Cotter SA, Lambert SR, Hutchinson AK, et al. Amblyopia preferred practice pattern. Ophthalmology. 2023;130(3):P136–P78. - PMC - PubMed
-
The objectives of this document are to identify characteristics and components of quality eye care for amblyopia based on the best available scientific data and randomized clinical trials.
-
- Leet MP, Bear MF, Gaier ED. Metaplasticity: a key to visual recovery from amblyopia in adulthood? Curr Opin Ophthalmol. 2022;33:512–518. - PMC - PubMed
-
This article provides an overview of research on metaplasticity in the visual system, and describes how the principles of metaplasticity may be employed to promote recovery from amblyopia in adulthood.
-
- Mitchell DE, Maurer D. Critical periods in vision revisited. Ann Rev Vis Sci. 2022;8:291–321. - PubMed
-
This article presents a review of research on the timing of critical periods in the visual system as it relates to damage by or recovery from monocular and binocular deprivation.
References
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19(4):126–30 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

