P62 promotes FSH-induced antral follicle formation by directing degradation of ubiquitinated WT1
- PMID: 38763964
- PMCID: PMC11102895
- DOI: 10.1007/s00018-024-05251-x
P62 promotes FSH-induced antral follicle formation by directing degradation of ubiquitinated WT1
Abstract
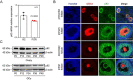
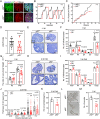
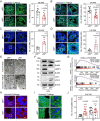
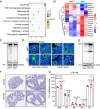
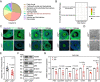
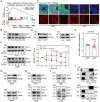
In females, the pathophysiological mechanism of poor ovarian response (POR) is not fully understood. Considering the expression level of p62 was significantly reduced in the granulosa cells (GCs) of POR patients, this study focused on identifying the role of the selective autophagy receptor p62 in conducting the effect of follicle-stimulating hormone (FSH) on antral follicles (AFs) formation in female mice. The results showed that p62 in GCs was FSH responsive and that its level increased to a peak and then decreased time-dependently either in ovaries or in GCs after gonadotropin induction in vivo. GC-specific deletion of p62 resulted in subfertility, a significantly reduced number of AFs and irregular estrous cycles, which were same as pathophysiological symptom of POR. By conducting mass spectrum analysis, we found the ubiquitination of proteins was decreased, and autophagic flux was blocked in GCs. Specifically, the level of nonubiquitinated Wilms tumor 1 homolog (WT1), a transcription factor and negative controller of GC differentiation, increased steadily. Co-IP results showed that p62 deletion increased the level of ubiquitin-specific peptidase 5 (USP5), which blocked the ubiquitination of WT1. Furthermore, a joint analysis of RNA-seq and the spatial transcriptome sequencing data showed the expression of steroid metabolic genes and FSH receptors pivotal for GCs differentiation decreased unanimously. Accordingly, the accumulation of WT1 in GCs deficient of p62 decreased steroid hormone levels and reduced FSH responsiveness, while the availability of p62 in GCs simultaneously ensured the degradation of WT1 through the ubiquitin‒proteasome system and autophagolysosomal system. Therefore, p62 in GCs participates in GC differentiation and AF formation in FSH induction by dynamically controlling the degradation of WT1. The findings of the study contributes to further study the pathology of POR.
Keywords: Antral follicles formation; FSH; Granulosa cell differentiation; Ubiquitinatized WT1; p62.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

Similar articles
-
LSD1 promotes the FSH responsive follicle formation by regulating autophagy and repressing Wt1 in the granulosa cells.Sci Bull (Beijing). 2024 Apr 30;69(8):1122-1136. doi: 10.1016/j.scib.2024.01.015. Epub 2024 Jan 17. Sci Bull (Beijing). 2024. PMID: 38302330
-
Epg5 deficiency leads to primary ovarian insufficiency due to WT1 accumulation in mouse granulosa cells.Autophagy. 2023 Feb;19(2):644-659. doi: 10.1080/15548627.2022.2094671. Epub 2022 Jul 27. Autophagy. 2023. PMID: 35786405 Free PMC article.
-
WT1 suppresses follicle-stimulating hormone-induced progesterone secretion by regulating ERK1/2 pathway in chicken preovulatory granulosa cells.Gene. 2022 Feb 20;812:146097. doi: 10.1016/j.gene.2021.146097. Epub 2021 Dec 11. Gene. 2022. PMID: 34902510
-
Review: Roles of follicle-stimulating hormone in preantral folliculogenesis of domestic animals: what can we learn from model species and where do we go from here?Animal. 2023 May;17 Suppl 1:100743. doi: 10.1016/j.animal.2023.100743. Animal. 2023. PMID: 37567683 Review.
-
ERβ Regulation of Gonadotropin Responses during Folliculogenesis.Int J Mol Sci. 2021 Sep 26;22(19):10348. doi: 10.3390/ijms221910348. Int J Mol Sci. 2021. PMID: 34638689 Free PMC article. Review.
Cited by
-
USP5 inhibition via bone marrow-targeted engineered exosomes for myeloproliferative neoplasms therapy.J Nanobiotechnology. 2025 Jul 11;23(1):501. doi: 10.1186/s12951-025-03588-4. J Nanobiotechnology. 2025. PMID: 40646529 Free PMC article.
-
Role of AMP-Activated Protein Kinase (AMPK) in Female Reproduction: A Review.Int J Mol Sci. 2025 Jul 16;26(14):6833. doi: 10.3390/ijms26146833. Int J Mol Sci. 2025. PMID: 40725074 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

