Stem cell exosome-loaded Gelfoam improves locomotor dysfunction and neuropathic pain in a rat model of spinal cord injury
- PMID: 38764049
- PMCID: PMC11103960
- DOI: 10.1186/s13287-024-03758-5
Stem cell exosome-loaded Gelfoam improves locomotor dysfunction and neuropathic pain in a rat model of spinal cord injury
Abstract
Background: Spinal cord injury (SCI) is a debilitating illness in humans that causes permanent loss of movement or sensation. To treat SCI, exosomes, with their unique benefits, can circumvent limitations through direct stem cell transplantation. Therefore, we utilized Gelfoam encapsulated with exosomes derived from human umbilical cord mesenchymal stem cells (HucMSC-EX) in a rat SCI model.
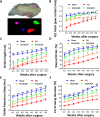
Methods: SCI model was established through hemisection surgery in T9 spinal cord of female Sprague-Dawley rats. Exosome-loaded Gelfoam was implanted into the lesion site. An in vivo uptake assay using labeled exosomes was conducted on day 3 post-implantation. Locomotor functions and gait analyses were assessed using Basso-Beattie-Bresnahan (BBB) locomotor rating scale and DigiGait Imaging System from weeks 1 to 8. Nociceptive responses were evaluated through von Frey filament and noxious radiant heat tests. The therapeutic effects and potential mechanisms were analyzed using Western blotting and immunofluorescence staining at week 8 post-SCI.
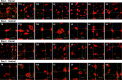
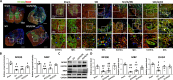
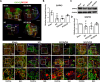
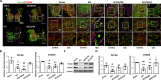
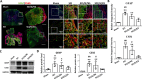
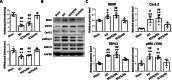
Results: For the in vivo exosome uptake assay, we observed the uptake of labeled exosomes by NeuN+, Iba1+, GFAP+, and OLIG2+ cells around the injured area. Exosome treatment consistently increased the BBB score from 1 to 8 weeks compared with the Gelfoam-saline and SCI control groups. Additionally, exosome treatment significantly improved gait abnormalities including right-to-left hind paw contact area ratio, stance/stride, stride length, stride frequency, and swing duration, validating motor function recovery. Immunostaining and Western blotting revealed high expression of NF200, MBP, GAP43, synaptophysin, and PSD95 in exosome treatment group, indicating the promotion of nerve regeneration, remyelination, and synapse formation. Interestingly, exosome treatment reduced SCI-induced upregulation of GFAP and CSPG. Furthermore, levels of Bax, p75NTR, Iba1, and iNOS were reduced around the injured area, suggesting anti-inflammatory and anti-apoptotic effects. Moreover, exosome treatment alleviated SCI-induced pain behaviors and reduced pain-associated proteins (BDNF, TRPV1, and Cav3.2). Exosomal miRNA analysis revealed several promising therapeutic miRNAs. The cell culture study also confirmed the neurotrophic effect of HucMSCs-EX.
Conclusion: Implantation of HucMSCs-EX-encapsulated Gelfoam improves SCI-induced motor dysfunction and neuropathic pain, possibly through its capabilities in nerve regeneration, remyelination, anti-inflammation, and anti-apoptosis. Overall, exosomes could serve as a promising therapeutic alternative for SCI treatment.
Keywords: And neuropathic pain; Exosomes; Glial scar; Locomotory function; Nerve regeneration; Spinal cord injury; Synapse formation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

