Adipose stem cells-derived small extracellular vesicles transport Thrombospondin 1 cargo to promote insulin resistance in gestational diabetes mellitus
- PMID: 38764083
- PMCID: PMC11103858
- DOI: 10.1186/s13098-024-01276-1
Adipose stem cells-derived small extracellular vesicles transport Thrombospondin 1 cargo to promote insulin resistance in gestational diabetes mellitus
Abstract
Background: Gestational diabetes mellitus (GDM) is a highly prevalent disease and poses a significant risk to the health of pregnant women. Abdominal adipose tissue (AT) contributes to insulin resistance (IR) associated with GDM. However, the underlying mechanisms remain unclear.
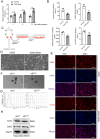
Methods: In this study, we developed a mouse model of GDM by subjecting mice to a high-fat diet. We collected adipose-derived stem cells (ADSCs) from the abdominal and inguinal regions and examined their role in inducing IR in normal tissues through the secretion of small extracellular vesicles (sEVs). The sEVs derived from ADSCs isolated from GDM mice (ADSC/GDM) were found to inhibit cell viability and insulin sensitivity in AML12, a normal mouse liver cell line.
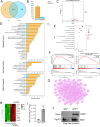
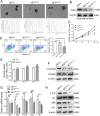
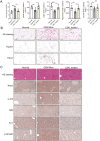
Results: Through proteomic analysis, we identified high levels of the thrombospondin 1 (Thbs1) protein in the sEVs derived from ADSC/GDM. Subsequent overexpression of Thbs1 protein in AML12 cells demonstrated similar IR as observed with ADSC/GDM-derived sEVs. Mechanistically, the Thbs1 protein within the sEVs interacted with CD36 and transforming growth factor (Tgf) β receptors in AML12 cells, leading to the activation of Tgfβ/Smad2 signaling. Furthermore, the administration of LSKL, an antagonistic peptide targeting Thbs1, suppressed Thbs1 expression in ADSC/GDM-derived sEVs, thereby restoring insulin sensitivity in AML12 cells and GDM mice in vivo.
Conclusions: These findings shed light on the intercellular transmission mechanism through which ADSCs influence hepatic insulin sensitivity and underscore the therapeutic potential of targeting the Thbs1 protein within sEVs.
Keywords: Adipose-derived stem cells; Gestational diabetes mellitus; Insulin resistance; Small extracellular vesicles; Thrombospondin 1.
© 2024. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures



References
-
- Geach T. Diabetes: a metabolomic signature to predict the transition from GDM to T2DM. Nat Rev Endocrinol. 2016;12(9):498. - PubMed
Grants and funding
- No. PWZxq2022-15/the Discipline Group Construction Program of the Health Bureau of Shanghai Pudong in China
- No. PWZxq2022-15/the Discipline Group Construction Program of the Health Bureau of Shanghai Pudong in China
- No. PKJ2021-Y30/Fund of Shanghai Pudong New Area Science and Technology Commission in China
LinkOut - more resources
Full Text Sources
Miscellaneous

