Comparative effects of progestin-based combination therapy for endometrial cancer or atypical endometrial hyperplasia: a systematic review and network meta-analysis
- PMID: 38764577
- PMCID: PMC11099254
- DOI: 10.3389/fonc.2024.1391546
Comparative effects of progestin-based combination therapy for endometrial cancer or atypical endometrial hyperplasia: a systematic review and network meta-analysis
Abstract
Objectives: The objective of this network meta-analysis is to systematically compare the efficacy of diverse progestin-based combination regimens in treating patients diagnosed with endometrial cancer or atypical endometrial hyperplasia. The primary goal is to discern the optimal combination treatment regimen through a comprehensive examination of their respective effectiveness.
Methods: We systematically searched four prominent databases: PubMed, Web of Science, Embase, and Cochrane Central Register of Controlled Trials, for randomized controlled trials addressing the efficacy of progestins or progestin combinations in the treatment of patients with endometrial cancer or atypical endometrial hyperplasia. The search spanned from the inception of these databases to December 2023. Key outcome indicators encompassed survival indices, criteria for assessing efficacy, as well as pregnancy and relapse rate. This study was registered in PROSPERO (CRD42024496311).
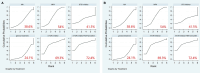
Results: From the 1,558 articles initially retrieved, we included 27 studies involving a total of 5,323 subjects in our analysis. The results of the network meta-analysis revealed that the mTOR inhibitor+megestrol acetate (MA)+tamoxifen regimen secured the top rank in maintaining stable disease (SD) (SUCRA=73.4%) and extending progression-free survival (PFS) (SUCRA=72.4%). Additionally, the progestin combined with tamoxifen regimen claimed the leading position in enhancing the partial response (PR) (SUCRA=75.2%) and prolonging overall survival (OS) (SUCRA=80%). The LNG-IUS-based dual progestin regimen emerged as the frontrunner in improving the complete response (CR) (SUCRA=98.7%), objective response rate (ORR) (SUCRA=99.1%), pregnancy rate (SUCRA=83.7%), and mitigating progression (SUCRA=8.0%) and relapse rate (SUCRA=47.4%). In terms of safety, The LNG-IUS-based dual progestin regimen had the lowest likelihood of adverse events (SUCRA=4.2%), while the mTOR inhibitor regimen (SUCRA=89.2%) and mTOR inbitor+MA+tamoxifen regimen (SUCRA=88.4%) had the highest likelihood of adverse events.
Conclusions: Patients diagnosed with endometrial cancer or atypical endometrial hyperplasia exhibited the most favorable prognosis when undergoing progestin combination therapy that included tamoxifen, mTOR inhibitor, or LNG-IUS. Notably, among these options, the LNG-IUS-based dual progestin regimen emerged as particularly promising for potential application.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42024496311.
Keywords: atypical endometrial hyperplasia; combination therapy; endometrial cancer; meta-analysis; progestin; systematic review.
Copyright © 2024 Cui, Zhao, She and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

