Long-Term Use of Rimegepant 75 mg for the Acute Treatment of Migraine is Associated with a Reduction in the Utilization of Select Analgesics and Antiemetics
- PMID: 38764606
- PMCID: PMC11102748
- DOI: 10.2147/JPR.S456006
Long-Term Use of Rimegepant 75 mg for the Acute Treatment of Migraine is Associated with a Reduction in the Utilization of Select Analgesics and Antiemetics
Abstract
Purpose: To examine use of concomitant analgesics and antiemetics during treatment with rimegepant in adults with migraine.
Patients and methods: This was a post hoc analysis of a long-term, open-label, safety study in adults with a history of 2-14 moderate or severe migraine attacks per month. Participants self-administered rimegepant 75 mg (1) up to once daily as needed (PRN) for 52 weeks or (2) every other day plus PRN (EOD+PRN) for 12 weeks. The PRN cohort was further divided based on baseline attack frequency, with PRN (2-8) and PRN (9-14) cohorts having a history of 2-8 or 9-14 attacks per month, respectively. Use of select analgesics and antiemetics was analyzed during a 30-day pre-treatment observation period (OP) and during rimegepant treatment.
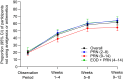
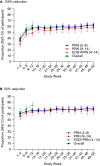
Results: Overall, 1800 rimegepant-treated participants (PRN n = 1514, EOD+PRN n = 286) were included in the analysis. Select analgesics or antiemetics were used by 80.1% of participants during the OP. Among 1441 participants using analgesics or antiemetics during the OP, the proportion who did not use any analgesics or antiemetics following initiation of rimegepant treatment increased during weeks 1-4 (36.9%), 5-8 (52.6%), and 9-12 (56.5%). The mean number of days per month using analgesics or antiemetics was also significantly reduced over time in all cohorts beginning at weeks 1-4 (P < 0.001 vs OP). This pattern of reduced analgesic or antiemetic use was consistent for all rimegepant cohorts, but was most pronounced in the EOD+PRN cohort in which 74.8% of participants reported ≥50% reduction in analgesic/antiemetic days at weeks 9-12. Reduction in use was maintained over time, with 61.3% of participants not using any analgesics or antiemetics during weeks 49-52 of PRN treatment.
Conclusion: Long-term treatment with oral rimegepant was associated with reduced analgesic and antiemetic use. Clinicaltrials.gov: NCT03266588.
Keywords: analgesics; antiemetics; clinical trial; migraine; rimegepant.
© 2024 Fullerton and Pixton.
Conflict of interest statement
Terence Fullerton and Glenn Pixton are full-time employees of, and own stock/options in, Pfizer. Glenn Pixton owns stock in Abbvie. The authors report no other conflicts of interest in this work.
Figures
References
-
- Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z; on behalf of Lifting The Burden: the Global Campaign against H. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137. doi:10.1186/s10194-020-01208-0 - DOI - PMC - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

