Temperature-Induced Luminescence Intensity Fluctuation of Protein-Protected Copper Nanoclusters: Role of Scaffold Conformation vs Nonradiative Transition
- PMID: 38764622
- PMCID: PMC11097160
- DOI: 10.1021/acsomega.4c02223
Temperature-Induced Luminescence Intensity Fluctuation of Protein-Protected Copper Nanoclusters: Role of Scaffold Conformation vs Nonradiative Transition
Abstract
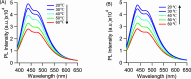
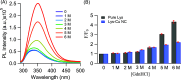
Protein-scaffolded atomically precise metal nanoclusters (NCs) have emerged as a promising class of biofriendly nanoprobes at the forefront of modern research, particularly in the area of sensing. The photoluminescence (PL) intensity of several nanoclusters showed a systematic temperature-dependent fluctuation, but the mechanism remains ambiguous and is poorly understood. We tried to shed some light on this mechanistic aspect by testing a couple of hypotheses: (i) conformational fluctuation of the protein scaffold-mediated PL intensity fluctuation and (ii) PL intensity fluctuation due to the variation in the radiative and nonradiative transition rates. Herein, the PL intensity of the lysozyme-capped copper nanocluster (Lys-Cu NC) showed excellent temperature dependency; upon increasing the temperature, the PL intensity gradually decreased. However, contrasting effects can be seen when the nanocluster is exposed to a chemical denaturant (guanidine hydrochloride (GdnHCl)); the PL intensity increased with the increase in the GdnHCl concentration due to the change in the ionic strength of the medium. This discrepancy clearly suggests that the thermal PL intensity fluctuation cannot be explained by a change in the scaffold conformation. Furthermore, upon closer investigation, we observed a 2-fold increase in the nonradiative decay rate of the Lys-Cu NC at the elevated temperature, which could reasonably explain the decrease in the PL intensity of the nanocluster at the higher temperature. Additionally, from the result, it was evident that the protein scaffold-metal core interaction played a key role here in stabilizing each other; hence, the scaffold structure remained unaffected even in the presence of chemical denaturants.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Matus M. F.; Häkkinen H. Understanding Ligand-Protected Noble Metal Nanoclusters at Work. Nat. Rev. Mater. 2023, 8, 372–389. 10.1038/s41578-023-00537-1. - DOI
-
- Das N. K.; Ghosh S.; Priya A.; Datta S.; Mukherjee S. Luminescent Copper Nanoclusters as a Specific Cell-Imaging Probe and a Selective Metal Ion Sensor. J. Phys. Chem. C 2015, 119 (43), 24657–24664. 10.1021/acs.jpcc.5b08123. - DOI
-
- Sebastian A.; Aarya; Sarangi B. R.; Sen Mojumdar S. Lysozyme Protected Copper Nano-Cluster: A Photo-Switch for the Selective Sensing of Fe2+. J. Photochem. Photobiol. A 2023, 436, 11437810.1016/j.jphotochem.2022.114378. - DOI
LinkOut - more resources
Full Text Sources
