The severity of meiotic aneuploidy is associated with altered morphokinetic variables of mouse oocyte maturation
- PMID: 38764910
- PMCID: PMC11099657
- DOI: 10.1093/hropen/hoae023
The severity of meiotic aneuploidy is associated with altered morphokinetic variables of mouse oocyte maturation
Abstract
Study question: Is there an association between morphokinetic variables of meiotic maturation and the severity of aneuploidy following in vitro maturation (IVM) in the mouse?
Summary answer: The severity of meiotic aneuploidy correlates with an extended time to first polar body extrusion (tPB1) and duration of meiosis I (dMI).
What is known already: Morphokinetic variables measured using time-lapse technology allow for the non-invasive evaluation of preimplantation embryo development within clinical assisted reproductive technology (ART). We recently applied this technology to monitor meiotic progression during IVM of mouse gametes. Whether there is a relationship between morphokinetic variables of meiotic progression and aneuploidy in the resulting egg has not been systematically examined at the resolution of specific chromosomes. Next-generation sequencing (NGS) is a robust clinical tool for determining aneuploidy status and has been reverse-translated in mouse blastocysts and oocytes. Therefore, we harnessed the technologies of time-lapse imaging and NGS to determine the relationship between the morphokinetics of meiotic progression and egg aneuploidy.
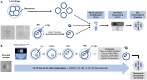
Study design size duration: Cumulus-oocyte complexes were collected from large antral follicles from hyperstimulated CD-1 mice. Cumulus cells were removed, and spontaneous IVM was performed in the absence or presence of two doses of Nocodazole (25 or 50 nM) to induce a spectrum of spindle abnormalities and chromosome segregation errors during oocyte meiosis. Comprehensive chromosome screening was then performed in the resulting eggs, and morphokinetic variables and ploidy status were compared across experimental groups (control, n = 11; 25 nM Nocodazole, n = 13; 50 nM Nocodazole, n = 23).
Participants/materials setting methods: We monitored IVM in mouse oocytes using time-lapse microscopy for 16 h, and time to germinal vesicle breakdown (tGVBD), tPB1, and dMI were analyzed. Following IVM, comprehensive chromosome screening was performed on the eggs and their matched first polar bodies via adaptation of an NGS-based preimplantation genetic testing for aneuploidy (PGT-A) assay. Bioinformatics analysis was performed to align reads to the mouse genome and determine copy number-based predictions of aneuploidy. The concordance of each polar body-egg pair (reciprocal errors) was used to validate the results. Ploidy status was categorized as euploid, 1-3 chromosomal segregation errors, or ≥4 chromosomal segregation errors. Additionally, aneuploidy due to premature separation of sister chromatids (PSSC) versus non-disjunction (NDJ) was distinguished.
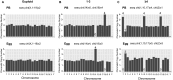
Main results and the role of chance: We applied and validated state-of-the-art NGS technology to screen aneuploidy in individual mouse eggs and matched polar bodies at the chromosome-specific level. By performing IVM in the presence of different doses of Nocodazole, we induced a range of aneuploidy. No aneuploidy was observed in the absence of Nocodazole (0/11), whereas IVM in the presence of 25 and 50 nM Nocodazole resulted in an aneuploidy incidence of 7.69% (1/13) and 82.61% (19/23), respectively. Of the aneuploid eggs, 5% (1/20) was due to PSSC, 65% (13/20) to NDJ, and the remainder to a combination of both. There was no relationship between ploidy status and tGVBD, but tPB1 and the dMI were both significantly prolonged in eggs with reciprocal aneuploidy events compared to the euploid eggs, and this scaled with the severity of aneuploidy. Eggs with ≥4 aneuploid chromosomes had the longest tPB1 and dMI (P < 0.0001), whereas eggs with one to three aneuploid chromosomes exhibited intermediate lengths of time (P < 0.0001).
Large scale data: N/A.
Limitations reasons for caution: We used Nocodazole in this study to disrupt the meiotic spindle and induce aneuploidy in mouse oocytes. Whether the association between morphokinetic variables of meiotic progression and the severity of aneuploidy occurs with other compounds that induce chromosome segregation errors remain to be investigated. In addition, unlike mouse oocytes, human IVM requires the presence of cumulus cells, which precludes visualization of morphokinetic variables of meiotic progression. Thus, our study may have limited direct clinical translatability.
Wider implications of the findings: We validated NGS in mouse eggs to detect aneuploidy at a chromosome-specific resolution which greatly improves the utility of the mouse model. With a tractable and validated model system for characterizing meiotic aneuploidy, investigations into the molecular mechanisms and factors which may influence aneuploidy can be further elaborated. Time-lapse analyses of morphokinetic variables of meiotic progression may be a useful non-invasive predictor of aneuploidy severity.
Study funding/competing interests: This work was supported by the Bill & Melinda Gates Foundation (INV-003385). Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The authors have no conflict of interest to disclose.
Keywords: aneuploidy; chromosomal abnormalities; in vitro maturation; meiosis; oocyte quality; time-lapse microscopy.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
This work was supported by the Bill & Melinda Gates Foundation (INV-003385). Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The authors have no conflict of interest to disclose.The authors have no conflict of interest to disclose.
Figures



Similar articles
-
Morphokinetic parameters of mouse oocyte meiotic maturation and cumulus expansion are not affected by reproductive age or ploidy status.J Assist Reprod Genet. 2023 May;40(5):1197-1213. doi: 10.1007/s10815-023-02779-y. Epub 2023 Apr 4. J Assist Reprod Genet. 2023. PMID: 37012451 Free PMC article.
-
Quantitative morphokinetic parameters identify novel dynamics of oocyte meiotic maturation and cumulus expansion†.Biol Reprod. 2022 Oct 11;107(4):1097-1112. doi: 10.1093/biolre/ioac139. Biol Reprod. 2022. PMID: 35810327 Free PMC article.
-
Aneuploidy in oocytes from women of advanced maternal age: analysis of the causal meiotic errors and impact on embryo development.Hum Reprod. 2023 Dec 4;38(12):2526-2535. doi: 10.1093/humrep/dead201. Hum Reprod. 2023. PMID: 37814912 Clinical Trial.
-
Morphological and morphokinetic associations with aneuploidy: a systematic review and meta-analysis.Hum Reprod Update. 2022 Aug 25;28(5):656-686. doi: 10.1093/humupd/dmac022. Hum Reprod Update. 2022. PMID: 35613016
-
Aneuploidy in human eggs: contributions of the meiotic spindle.Biochem Soc Trans. 2021 Feb 26;49(1):107-118. doi: 10.1042/BST20200043. Biochem Soc Trans. 2021. PMID: 33449109 Free PMC article. Review.
Cited by
-
Environmental Stress-Induced Alterations in Embryo Developmental Morphokinetics.J Xenobiot. 2024 Oct 21;14(4):1613-1637. doi: 10.3390/jox14040087. J Xenobiot. 2024. PMID: 39449428 Free PMC article. Review.
-
Preimplantation development analysis of aneuploid embryos with different chromosomal abnormalities.Heliyon. 2024 Nov 26;10(23):e40686. doi: 10.1016/j.heliyon.2024.e40686. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39687119 Free PMC article.
References
-
- Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CF.. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod Biomed Online 2013;26:477–485. - PubMed
-
- Decordier I, Cundari E, Kirsch-Volders M.. Survival of aneuploid, micronucleated and/or polyploid cells: crosstalk between ploidy control and apoptosis. Mutat Res 2008;651:30–39. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
