Modulation of neuronal dynamics by sustained and activity-dependent continuous-wave near-infrared laser stimulation
- PMID: 38764942
- PMCID: PMC11100521
- DOI: 10.1117/1.NPh.11.2.024308
Modulation of neuronal dynamics by sustained and activity-dependent continuous-wave near-infrared laser stimulation
Abstract
Significance: Near-infrared laser illumination is a non-invasive alternative/complement to classical stimulation methods in neuroscience but the mechanisms underlying its action on neuronal dynamics remain unclear. Most studies deal with high-frequency pulsed protocols and stationary characterizations disregarding the dynamic modulatory effect of sustained and activity-dependent stimulation. The understanding of such modulation and its widespread dissemination can help to develop specific interventions for research applications and treatments for neural disorders.
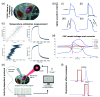
Aim: We quantified the effect of continuous-wave near-infrared (CW-NIR) laser illumination on single neuron dynamics using sustained stimulation and an open-source activity-dependent protocol to identify the biophysical mechanisms underlying this modulation and its time course.
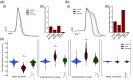
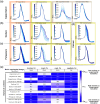
Approach: We characterized the effect by simultaneously performing long intracellular recordings of membrane potential while delivering sustained and closed-loop CW-NIR laser stimulation. We used waveform metrics and conductance-based models to assess the role of specific biophysical candidates on the modulation.
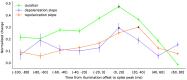
Results: We show that CW-NIR sustained illumination asymmetrically accelerates action potential dynamics and the spiking rate on single neurons, while closed-loop stimulation unveils its action at different phases of the neuron dynamics. Our model study points out the action of CW-NIR on specific ionic-channels and the key role of temperature on channel properties to explain the modulatory effect.
Conclusions: Both sustained and activity-dependent CW-NIR stimulation effectively modulate neuronal dynamics by a combination of biophysical mechanisms. Our open-source protocols can help to disseminate this non-invasive optical stimulation in novel research and clinical applications.
Keywords: activity-dependent optical stimulation; computational models; continuous-wave NIR laser neurotechnology; ionic channel dynamics; neuronal excitability; open-source techniques.
© 2024 The Authors.
Figures



References
-
- Carter M., Shieh J. C., Guide to Research Techniques in Neuroscience, Academic Press; (2015).
LinkOut - more resources
Full Text Sources
Miscellaneous

