Case report: Rapid resolution of grade IV ICANS after first line intrathecal chemotherapy with methotrexate, cytarabine and dexamethasone
- PMID: 38765003
- PMCID: PMC11099209
- DOI: 10.3389/fimmu.2024.1380451
Case report: Rapid resolution of grade IV ICANS after first line intrathecal chemotherapy with methotrexate, cytarabine and dexamethasone
Abstract
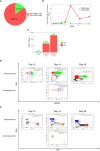
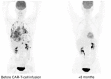
Corticosteroid therapy is the mainstay of immune effector cell-associated neurotoxicity syndrome (ICANS) management, although its use has been associated with worse overall survival (OS) and progression-free survival (PFS) after chimeric antigen receptor T-cell (CAR-T cell) therapy. Many options are being investigated for prophylaxis and management. Accumulating evidence supports the use of intrathecal (IT) chemotherapy for the management of high-grade ICANS. Here, we describe a case of a patient with stage IV Primary mediastinal B-cell lymphoma (PMBCL) successfully treated with IT methotrexate, cytarabine, and dexamethasone as first-line therapy for CD19 CAR-T cell-associated grade IV ICANS. The stable and rapid resolution of ICANS to grade 0 allowed us to discontinue systemic corticosteroid use, avoiding CAR-T cells ablation and ensuring preservation of CAR-T cell function. The described patient achieved a complete radiologic and clinical response to CD19 CAR-T cell therapy and remains disease-free after 9 months. This case demonstrates a promising example of how IT chemotherapy could be used as first-line treatment for the management of high-grade ICANS.
Keywords: CD19 CAR-T cells; ICANS; anakinra; corticosteroids; intrathecal chemotherapy.
Copyright © 2024 Katsin, Shman, Migas, Lutskovich, Serada, Khalankova, Kostina and Dubovik.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

