The diagnosis and management of systemic autoimmune rheumatic disease-related interstitial lung disease: British Society for Rheumatology guideline scope
- PMID: 38765189
- PMCID: PMC11101284
- DOI: 10.1093/rap/rkae056
The diagnosis and management of systemic autoimmune rheumatic disease-related interstitial lung disease: British Society for Rheumatology guideline scope
Erratum in
-
Correction to: The diagnosis and management of systemic autoimmune rheumatic disease-related interstitial lung disease: British Society for Rheumatology guideline scope.Rheumatol Adv Pract. 2024 Jul 27;8(3):rkae085. doi: 10.1093/rap/rkae085. eCollection 2024. Rheumatol Adv Pract. 2024. PMID: 39071088 Free PMC article.
Abstract
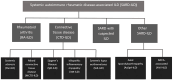
Interstitial lung disease (ILD) is a significant complication of many systemic autoimmune rheumatic diseases (SARDs), although the clinical presentation, severity and outlook may vary widely between individuals. Despite the prevalence, there are no specific guidelines addressing the issue of screening, diagnosis and management of ILD across this diverse group. Guidelines from the ACR and EULAR are expected, but there is a need for UK-specific guidelines that consider the framework of the UK National Health Service, local licensing and funding strategies. This article outlines the intended scope for the British Society for Rheumatology guideline on the diagnosis and management of SARD-ILD developed by the guideline working group. It specifically identifies the SARDs for consideration, alongside the overarching principles for which systematic review will be conducted. Expert consensus will be produced based on the most up-to-date available evidence for inclusion within the final guideline. Key issues to be addressed include recommendations for screening of ILD, identifying the methodology and frequency of monitoring and pharmacological and non-pharmacological management. The guideline will be developed according to methods and processes outlined in Creating Clinical Guidelines: British Society for Rheumatology Protocol version 5.1.
Keywords: UK; connective tissue disease; guideline; interstitial lung disease; scope.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
References
-
- Maher TM, Tudor VA, Saunders P. et al. Rituximab versus intravenous cyclophosphamide in patients with connective tissue disease-associated interstitial lung disease in the UK (RECITAL): a double-blind, double-dummy, randomised, controlled, phase 2b trial. Lancet Respir Med 2023;11:45–54. - PubMed
-
- Khanna D, Lin CJF, Furst DE. et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2020;8:963–74. - PubMed
-
- Distler O, Highland KB, Gahlemann M. et al. Nintedanib for systemic sclerosis–associated interstitial lung disease. N Engl J Med 2019;380:2518–28. - PubMed
-
- National Institute for Health and Care Excellence. Nintedanib for treating progressive fibrosing interstitial lung diseases. London: National Institute for Health and Care Excellence, 2021. https://www.nice.org.uk/guidance/ta747 (1 November 2023, date last accessed).
Publication types
LinkOut - more resources
Full Text Sources