A descriptive study of new drug approvals during 2017-2021 and disease morbidity and mortality patterns in India
- PMID: 38765552
- PMCID: PMC11101002
- DOI: 10.4103/picr.picr_109_23
A descriptive study of new drug approvals during 2017-2021 and disease morbidity and mortality patterns in India
Abstract
Aim: Studies show the presence of a mismatch between drug research and disease burden. A study conducted in the European Union found that new drug development was restricted to certain diseases. A study of biosimilar approvals in India found that 87% of drugs were for treating noncommunicable diseases. This study aimed to determine the new drugs approved in India from 2017 to 2021 and the top ten causes of morbidity and mortality and detect the presence of any discordance between these.
Methods: A descriptive study was conducted using data on new drug approvals accessed from the Central Drugs Standard Control Organization website. The top ten causes of mortality and morbidity in India from 2015 to 2019 were identified from the Global Burden of Diseases database. Descriptive statistics were used to compare the drug approvals and the leading diseases.
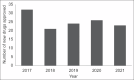
Results: One hundred twenty-six drugs were approved during the study period. Antineoplastic drugs constituted 19.84% of the approvals, antimicrobials 18.25%, and cardiovascular drugs 9.52%. Ischemic heart disease and chronic obstructive pulmonary disease were the two leading causes of morbidity and mortality. Diarrheal diseases, lower respiratory tract infection, and drug-susceptible tuberculosis were among the top ten causes. Ten antibacterials, including four antitubercular drugs, were approved during this period. Two drugs were approved for rare diseases.
Conclusion: Our study showed that the drugs approved were largely in line with the prevalent disease burden, and there was no significant discordance observed. Some diseases, such as ischemic stroke/intracranial hemorrhage, require further efforts in bringing forth newer pharmacotherapy options.
Keywords: Drug approval; investigational new drugs; morbidity; mortality; noncommunicable diseases.
Copyright: © 2023 Perspectives in Clinical Research.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
A comparative study of cancer drug approvals in india and high-income countries.J Cancer Policy. 2022 Sep;33:100349. doi: 10.1016/j.jcpo.2022.100349. Epub 2022 Jul 25. J Cancer Policy. 2022. PMID: 35902067
-
Drug lag for cardiovascular drug approvals in India compared with the US and EU approvals.Indian Heart J. 2013 Jan-Feb;65(1):24-9. doi: 10.1016/j.ihj.2012.12.024. Epub 2012 Dec 27. Indian Heart J. 2013. PMID: 23438609 Free PMC article.
-
Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study.Lancet. 1997 May 17;349(9063):1436-42. doi: 10.1016/S0140-6736(96)07495-8. Lancet. 1997. PMID: 9164317
-
Review of novel and supplemental approvals of the targeted cancer drugs by the Food and Drug Administration in 2021.J Oncol Pharm Pract. 2023 Jan;29(1):191-207. doi: 10.1177/10781552221112015. Epub 2022 Jul 6. J Oncol Pharm Pract. 2023. PMID: 35793068 Review.
-
Assessment of the Clinical Benefit of Cancer Drugs Receiving Accelerated Approval.JAMA Intern Med. 2019 Jul 1;179(7):906-913. doi: 10.1001/jamainternmed.2019.0462. JAMA Intern Med. 2019. PMID: 31135808 Free PMC article. Review.
References
-
- George S, Chandran AB, Nadh PO, Apurva KH. Is drug development in India responsive to the disease burden? EPW. 2018;53:51–7.
-
- Charan J, Dhanani JV, Doshi MS, Reljic T, Tsalatsanis A, Kumar A. Drug approval in India does not match the disease burden: A cross-sectional study. J Pharmacol Pharmacother. 2018;9:6–10.
-
- Trouiller P, Olliaro P, Torreele E, Orbinski J, Laing R, Ford N. Drug development for neglected diseases: A deficient market and a public-health policy failure. Lancet. 2002;359:2188–94. - PubMed
LinkOut - more resources
Full Text Sources