"Eculizumab First" in the Management of Posttransplant Thrombotic Microangiopathy
- PMID: 38765562
- PMCID: PMC11101752
- DOI: 10.1016/j.ekir.2024.01.013
"Eculizumab First" in the Management of Posttransplant Thrombotic Microangiopathy
Abstract
Introduction: Posttransplant thrombotic microangiopathy (PT-TMA) is an uncommon event that characterizes approximately 3% to 14% of kidney transplants (KTs), and that is associated with a higher risk of delayed graft function and graft loss. PT-TMA occurs more frequently within the first 3 months after transplant and can be a manifestation of de novo disease or the recurrence of previous atypical hemolytic uremic syndrome (aHUS). Abnormalities in complement regulation genes could explain the increased susceptibility of some patients to PT-TMA. Eculizumab is a humanized monoclonal antibody that inhibits the formation of the membrane attack complex C5b-9. The aim of this study is to evaluate the efficacy of eculizumab as treatment for PT-TMA.
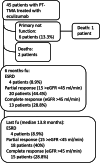
Methods: We retrospectively analyzed clinical records of 45 KT patients who received eculizumab immediately after the clinical diagnosis of PT-TMA.
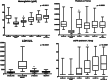
Results: Kidney biopsy was performed in 91.1% of patients, and complement genetic study was performed in 64.4%. Of the kidney biopsies, 85.4% showed signs of TMA; genetic analysis revealed 1 pathogenetic variant, 2 variants of uncertain significance, 1 likely benign variant, 8 risk polymorphisms, and 27 risk haplotypes. After 2 weeks from the treatment starting, hemoglobin and platelets significantly increased. A remarkable improvement in kidney function was also observed. After 6 months, 28.8% of patients had a complete renal recovery whereas 44.4% had a partial recovery.
Conclusion: This is, to our knowledge, the largest series of KT patients with PT-TMA treated with eculizumab. These data suggest that eculizumab is associated with a normalization of hemolysis indices and an important and progressive improvement of graft function.
Keywords: atypical hemolytic uremic syndrome; eculizumab; kidney transplant; thrombotic microangiopathy.
Figures


References
-
- Chiurchiu C., Ruggenenti P., Remuzzi G. Thrombotic microangiopathy in renal transplantation. Ann Transplant. 2002;7:28–33. - PubMed
LinkOut - more resources
Full Text Sources

