Pulmonary Hypertension With Interstitial Pneumonia: Initial Treatment Effectiveness and Severity in a Japan Registry
- PMID: 38765657
- PMCID: PMC11099821
- DOI: 10.1016/j.jacasi.2024.01.009
Pulmonary Hypertension With Interstitial Pneumonia: Initial Treatment Effectiveness and Severity in a Japan Registry
Abstract
Background: Recent guidelines discourage the use of pulmonary arterial hypertension (PAH)-targeted therapies in patients with pulmonary hypertension (PH) associated with respiratory diseases. Therefore, stratifications of the effectiveness of PAH-targeted therapies are important for this group.
Objectives: The authors aimed to identify phenotypes that might benefit from initial PAH-targeted therapies in patients with PH associated with interstitial pneumonia and combined pulmonary fibrosis and emphysema.
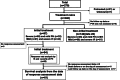
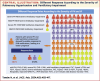
Methods: We categorized 270 patients with precapillary PH (192 interstitial pneumonia, 78 combined pulmonary fibrosis and emphysema) into severe and mild PH using a pulmonary vascular resistance of 5 WU. We investigated the prognostic factors and compared the prognoses of initial (within 2 months after diagnosis) and noninitial treatment groups, as well as responders (improvements in World Health Organization functional class, pulmonary vascular resistance, and 6-minute walk distance) and nonresponders.
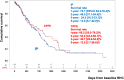
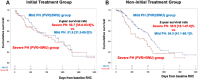
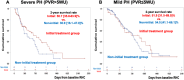
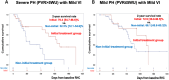
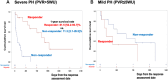
Results: Among 239 treatment-naive patients, 46.0% had severe PH, 51.8% had mild ventilatory impairment (VI), and 40.6% received initial treatment. In the severe PH with mild VI subgroup, the initial treatment group had a favorable prognosis compared with the noninitial treatment group. The response rate in this group was significantly higher than the others (48.2% vs 21.8%, ratio 2.21 [95% CI: 1.17-4.16]). In multivariate analysis, initial treatment was a better prognostic factor for severe PH but not for mild PH. Within the severe PH subgroup, responders had a favorable prognosis.
Conclusions: This study demonstrated an increased number of responders to initial PAH-targeted therapy, with a favorable prognosis in severe PH cases with mild VI. A survival benefit was not observed in mild PH cases. (Multi-institutional Prospective Registry in Pulmonary Hypertension associated with Respiratory Disease; UMIN000011541).
Keywords: interstitial pneumonia; multicenter registry; pulmonary hypertension; respiratory disease; ventilatory impairment.
© 2024 The Authors.
Conflict of interest statement
This study was supported by grants from Grant-in-Aid for Scientific Research (20FC1027, 23FC1031) from the Ministry of Health, Labour and Welfare of Japan, the Medical Research Fund of the Japan Medical Association (No. 16ek0109127h0002 and No. JP18lk1601003h0001), and the Nonprofit Organization Japan PH registry. Dr Tanabe has received remuneration from Nippon Shinyaku, Janssen Pharmaceutical K.K., and Bayer Yakuhin, and belongs to a department endowed by Nippon Shinyaku. Dr Kumamaru has received consultation fees from Mitsubishi-Tanabe Pharma Corp, and EPS Corp; speaker fee from Chugai Pharmaceutical Co, Ltd; and a research grant from Pfizer Japan Inc. Drs Kumamaru, Kinukawa, and Miyata are affiliated with the Department of Health Quality Assessment at the University of Tokyo, a social collaboration department supported by the National Clinical Database, Johnson & Johnson K.K., Nipro Corp, and Intuitive Surgical Sàrl. Dr Nishiyama has received remuneration from Nippon Shinyaku, Janssen Pharmaceutical K.K., and Boehringer Ingelheim. Dr Tsujino has received remuneration from Nippon Shinyaku and Janssen Pharmaceutical K.K., and belongs to a department endowed by Nippon Shinyaku, Mochida Pharmaceutical Co Ltd, Boehringer Ingelheim, Takeyama Co, Ltd, Kaneka Medics Co, and Medical System Network Co Ltd. Dr Inoue has received remuneration from Boehringer Ingelheim. Dr Hirata has received a research grant from Janssen Pharmaceutical K.K. Dr Kuwana has received remuneration from Nippon Shinyaku. Dr Handa has received a research grant from Fujifilm Corp and belongs to a department endowed by Teijin Pharma Ltd. Dr Taniguchi has received a research grant from Janssen Pharmaceutical K.K. Dr Matsubara has received remuneration from Nippon Shinyaku, Janssen Pharmaceutical K.K., Bayer Yakuhin, Mochida Pharmaceutical. Co Ltd, and Kaneka Medics Co; and research grants from Nippon Shinyaku, Janssen Pharmaceutical K.K., Mochida Pharmaceutical Co Ltd, and Insmed Incorporated. Dr Tatsumi has received remuneration from Janssen Pharmaceutical K.K. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


References
-
- Waxman A., Restrepo-Jaramillo R., Thenappan T., et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384:325–334. - PubMed
-
- Olsson K.M., Hoeper M.M., Pausch C., et al. Pulmonary vascular resistance predicts mortality in patients with pulmonary hypertension associated with interstitial lung disease: results from the COMPERA registry. Eur Respir J. 2021;58 - PubMed
LinkOut - more resources
Full Text Sources

