This is a preprint.
Associations Between Prenatal Vitamin D and Placental Gene Expression
- PMID: 38765981
- PMCID: PMC11100832
- DOI: 10.1101/2024.05.10.593571
Associations Between Prenatal Vitamin D and Placental Gene Expression
Update in
-
Associations Between Prenatal Vitamin D and Placental Gene Expression.J Nutr. 2024 Dec;154(12):3603-3614. doi: 10.1016/j.tjnut.2024.10.019. Epub 2024 Oct 12. J Nutr. 2024. PMID: 39401684 Free PMC article.
Abstract
Background: Vitamin D is a hormone regulating gene transcription. Prenatal vitamin D has been linked to immune and vascular function in the placenta, a key organ of pregnancy. To date, studies of vitamin D and placental gene expression have focused on a limited number of candidate genes. Transcriptome-wide RNA sequencing can provide a more complete representation of the placental effects of vitamin D.
Objective: We investigated the association between prenatal vitamin D levels and placental gene expression in a large, prospective pregnancy cohort.
Methods: Participants were recruited in Shelby County, Tennessee in the Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study. Vitamin D level (plasma total 25-hydroxyvitatmin D, [25(OH)D]) was measured at mid-pregnancy (16-28 weeks' gestation) and delivery. Placenta samples were collected at birth. RNA was isolated and sequenced. We identified differentially expressed genes (DEGs) using adjusted linear regression models. We also conducted weighted gene co-expression network analysis (WGCNA).
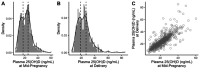
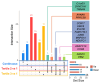
Results: The median 25(OH)D of participants was 21.8 ng/mL at mid-pregnancy (N=774, IQR: 15.4-26.5 ng/mL) and 23.6 ng/mL at delivery (N=753, IQR: 16.8-29.1 ng/mL). Placental expression of 25 DEGs was associated with 25(OH)D at mid-pregnancy, but no DEG was associated with 25(OH)D at delivery. DEGs were related to energy metabolism, cytoskeletal function, and RNA transcription. Using WGCNA, we identified 2 gene modules whose expression was associated with 25(OH)D at mid-pregnancy and 1 module associated with 25(OH)D at delivery. These modules were enriched for genes related to mitochondrial and cytoskeletal function, and were regulated by transcription factors including ARNT2, BHLHE40, FOSL2, JUND, and NFKB1.
Conclusions: Our results indicate that 25(OH)D during mid-pregnancy, but not at delivery, is associated with placental gene expression at birth. Future research is needed to investigate a potential role of vitamin D in programming placental mitochondrial metabolism, intracellular transport, and transcriptional regulation during pregnancy.
Figures

References
-
- Wagner CL, Hollis BW. The Implications of Vitamin D Status During Pregnancy on Mother and her Developing Child. Frontiers in Endocrinology [Internet] Frontiers Media SA; 2018. [cited 2024 Feb 15];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6127214/ - PMC - PubMed
-
- Wilson RL, Leviton AJ, Leemaqz SY, Anderson PH, Grieger JA, Grzeskowiak LE, Verburg PE, McCowan L, Dekker GA, Bianco-Miotto T, et al. Vitamin D levels in an Australian and New Zealand cohort and the association with pregnancy outcome. BMC Pregnancy and Childbirth [Internet] BMC; 2018. [cited 2024 Feb 16];18. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6011374/ - PMC - PubMed
-
- Fogacci S, Fogacci F, Banach M, Michos ED, Hernandez AV, Lip GYH, Blaha MJ, Toth PP, Borghi C, Cicero AFG. Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clinical Nutrition 2020;39:1742–52. - PubMed
-
- Milajerdi A, Abbasi F, Mousavi SM, Esmaillzadeh A. Maternal vitamin D status and risk of gestational diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Clin Nutr 2021;40:2576–86. - PubMed
-
- Zhao R, Zhou L, Wang S, Yin H, Yang X, Hao L. Effect of maternal vitamin D status on risk of adverse birth outcomes: a systematic review and dose–response meta-analysis of observational studies. Eur J Nutr 2022;61:2881–907. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
