This is a preprint.
Microglia regulate motor neuron plasticity via reciprocal fractalkine/adenosine signaling
- PMID: 38765982
- PMCID: PMC11100694
- DOI: 10.1101/2024.05.07.592939
Microglia regulate motor neuron plasticity via reciprocal fractalkine/adenosine signaling
Update in
-
Microglia regulate motor neuron plasticity via reciprocal fractalkine and adenosine signaling.Nat Commun. 2024 Nov 28;15(1):10349. doi: 10.1038/s41467-024-54619-x. Nat Commun. 2024. PMID: 39609435 Free PMC article.
Abstract
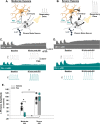
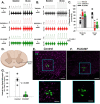
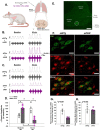
Microglia are innate CNS immune cells that play key roles in supporting key CNS functions including brain plasticity. We now report a previously unknown role for microglia in regulating neuroplasticity within spinal phrenic motor neurons, the neurons driving diaphragm contractions and breathing. We demonstrate that microglia regulate phrenic long-term facilitation (pLTF), a form of respiratory memory lasting hours after repetitive exposures to brief periods of low oxygen (acute intermittent hypoxia; AIH) via neuronal/microglial fractalkine signaling. AIH-induced pLTF is regulated by the balance between competing intracellular signaling cascades initiated by serotonin vs adenosine, respectively. Although brainstem raphe neurons release the relevant serotonin, the cellular source of adenosine is unknown. We tested a model in which hypoxia initiates fractalkine signaling between phrenic motor neurons and nearby microglia that triggers extracellular adenosine accumulation. With moderate AIH, phrenic motor neuron adenosine 2A receptor activation undermines serotonin-dominant pLTF; in contrast, severe AIH drives pLTF by a unique, adenosine-dominant mechanism. Phrenic motor neuron fractalkine knockdown, cervical spinal fractalkine receptor inhibition on nearby microglia, and microglial depletion enhance serotonin-dominant pLTF with moderate AIH but suppress adenosine-dominant pLTF with severe AIH. Thus, microglia play novel functions in the healthy spinal cord, regulating hypoxia-induced neuroplasticity within the motor neurons responsible for breathing.
Conflict of interest statement
ETHICS DECLARATIONS The authors declare no competing interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
