This is a preprint.
Dopey-dependent regulation of extracellular vesicles maintains neuronal morphology
- PMID: 38766017
- PMCID: PMC11100700
- DOI: 10.1101/2024.05.07.591898
Dopey-dependent regulation of extracellular vesicles maintains neuronal morphology
Update in
-
Dopey-dependent regulation of extracellular vesicles maintains neuronal morphology.Curr Biol. 2024 Nov 4;34(21):4920-4933.e11. doi: 10.1016/j.cub.2024.09.018. Epub 2024 Oct 7. Curr Biol. 2024. PMID: 39378880
Abstract
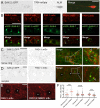
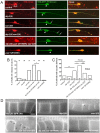
Mature neurons maintain their distinctive morphology for extended periods in adult life. Compared to developmental neurite outgrowth, axon guidance, and target selection, relatively little is known of mechanisms that maintain mature neuron morphology. Loss of function in C. elegans DIP-2, a member of the conserved lipid metabolic regulator Dip2 family, results in progressive overgrowth of neurites in adults. We find that dip-2 mutants display specific genetic interactions with sax-2, the C. elegans ortholog of Drosophila Furry and mammalian FRY. Combined loss of DIP-2 and SAX-2 results in severe disruption of neuronal morphology maintenance accompanied by increased release of neuronal extracellular vesicles (EVs). By screening for suppressors of dip-2 sax-2 double mutant defects we identified gain-of-function (gf) mutations in the conserved Dopey family protein PAD-1 and its associated phospholipid flippase TAT-5/ATP9A. In dip-2 sax-2 double mutants carrying either pad-1(gf) or tat-5(gf) mutation, EV release is reduced and neuronal morphology across multiple neuron types is restored to largely normal. PAD-1(gf) acts cell autonomously in neurons. The domain containing pad-1(gf) is essential for PAD-1 function, and PAD-1(gf) protein displays increased association with the plasma membrane and inhibits EV release. Our findings uncover a novel functional network of DIP-2, SAX-2, PAD-1, and TAT-5 that maintains morphology of neurons and other types of cells, shedding light on the mechanistic basis of neurological disorders involving human orthologs of these genes.
Keywords: DIP-2 lipid regulator; PAD-1/DOPEY; SAX-2/Fry; TAT-5 phospholipid flippase; endosomal trafficking; neuronal maintenance.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
