This is a preprint.
Neuroinflammatory Responses and Blood-Brain Barrier Injury in Chronic Alcohol Exposure: Role of Purinergic P2X7 Receptor Signaling
- PMID: 38766082
- PMCID: PMC11100971
- DOI: 10.21203/rs.3.rs-4350949/v1
Neuroinflammatory Responses and Blood-Brain Barrier Injury in Chronic Alcohol Exposure: Role of Purinergic P2X7 Receptor Signaling
Update in
-
Neuroinflammatory responses and blood-brain barrier injury in chronic alcohol exposure: role of purinergic P2 × 7 Receptor signaling.J Neuroinflammation. 2024 Sep 28;21(1):244. doi: 10.1186/s12974-024-03230-4. J Neuroinflammation. 2024. PMID: 39342243 Free PMC article.
Abstract
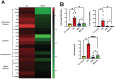
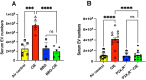
Alcohol consumption leads to neuroinflammation and blood-brain barrier (BBB) damage, resulting in neurological impairment. We previously demonstrated that ethanol-induced disruption of barrier function in human brain endothelial cells was associated with mitochondrial injury, increased ATP and extracellular vesicle (EV) release, and purinergic receptor P2X7R activation. Therefore, we aimed to evaluate the effect of P2X7r blockade on peripheral and neuro-inflammation in EtOH-exposed mice. In a chronic intermittent ethanol (CIE)-exposed mouse model, P2X7R was inhibited by two different methods: Brilliant Blue G (BBG) or gene knockout. We assessed blood ethanol concentration (BEC), plasma P2X7R and P-gp, number of extra-cellular vesicles (EV), serum ATP and EV-ATP levels. Brain microvessel gene expression and EV mtDNA copy numbers were measured by RT2 PCR array and digital PCR, respectively. A RT2 PCR array of brain microvessels revealed significant upregulation of proinflammatory genes involved in apoptosis, vasodilation, and platelet activation in CIE-exposed animals, which were decreased 15-50-fold in BBG-treated CIE-exposed animals. Plasma P-gp levels and serum P2X7R shedding were significantly increased in CIE-exposed animals. Pharmacological or genetic suppression of P2X7R decreased P2X7R shedding to levels equivalent to those in control group. The increase in EV number and EV-ATP content in the CIE-exposed mice was significantly reduced by P2X7R inhibition. CIE mice showed augmented EV-mtDNA copy numbers which were reduced in EVs after P2X7R inhibition or receptor knockout. These observations suggested that P2X7R signaling plays a critical role in ethanol-induced brain injury. Increased eATP, EV-ATP, EV numbers, and EV-mtDNA copy numbers highlight a new mechanism of brain injury during alcohol exposure via P2X7R and biomarkers of such damage. In this study, for the first time, we report the in vivo involvement of P2X7R signaling in CIE-induced brain injury.
Keywords: ATP; Blood-brain barrier; CIE; Extracellular vesicles; P2X7R.
Conflict of interest statement
Conflict of interest None of the authors has any potential financial conflict of interest related to this manuscript. Additional Declarations: No competing interests reported.
Figures






Similar articles
-
Neuroinflammatory responses and blood-brain barrier injury in chronic alcohol exposure: role of purinergic P2 × 7 Receptor signaling.J Neuroinflammation. 2024 Sep 28;21(1):244. doi: 10.1186/s12974-024-03230-4. J Neuroinflammation. 2024. PMID: 39342243 Free PMC article.
-
Alcohol and e-cigarette damage alveolar-epithelial barrier by activation of P2X7r and provoke brain endothelial injury via extracellular vesicles.Cell Commun Signal. 2024 Jan 15;22(1):39. doi: 10.1186/s12964-023-01461-1. Cell Commun Signal. 2024. PMID: 38225580 Free PMC article.
-
Alcohol and e-cigarette damage alveolar-epithelial barrier by activation of P2X7r and provoke brain endothelial injury via extracellular vesicles.Res Sq [Preprint]. 2023 Nov 14:rs.3.rs-3552555. doi: 10.21203/rs.3.rs-3552555/v1. Res Sq. 2023. Update in: Cell Commun Signal. 2024 Jan 15;22(1):39. doi: 10.1186/s12964-023-01461-1. PMID: 38014253 Free PMC article. Updated. Preprint.
-
Recent Advances in the Development of Antidepressants Targeting the Purinergic P2X7 Receptor.Curr Med Chem. 2023;30(2):164-177. doi: 10.2174/0929867329666220629141418. Curr Med Chem. 2023. PMID: 35770396 Review.
-
Role of ATP in Extracellular Vesicle Biogenesis and Dynamics.Front Pharmacol. 2021 Mar 15;12:654023. doi: 10.3389/fphar.2021.654023. eCollection 2021. Front Pharmacol. 2021. PMID: 33790800 Free PMC article. Review.
References
-
- Global status report on alcohol and health. 2018. In. Edited by Poznyak V, D. R.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

