This is a preprint.
The Glucose Transporter 5 Enhances CAR-T Cell Metabolic Function and Anti-tumour Durability
- PMID: 38766088
- PMCID: PMC11100898
- DOI: 10.21203/rs.3.rs-4342820/v1
The Glucose Transporter 5 Enhances CAR-T Cell Metabolic Function and Anti-tumour Durability
Abstract
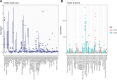
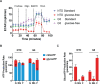
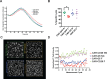
Activated T cells undergo a metabolic shift to aerobic glycolysis to support the energetic demands of proliferation, differentiation, and cytolytic function. Transmembrane glucose flux is facilitated by glucose transporters (GLUT) that play a vital role in T cell metabolic reprogramming and anti-tumour function. GLUT isoforms are regulated at the level of expression and subcellular distribution. GLUTs also display preferential selectivity for carbohydrate macronutrients including glucose, galactose, and fructose. GLUT5, which selectively transports fructose over glucose, has never been explored as a genetic engineering strategy to enhance CAR-T cells in fructose-rich tumour environments. Fructose levels are significantly elevated in the bone marrow and the plasma of acute myeloid leukaemia (AML) patients. Here, we demonstrate that the expression of wild-type GLUT5 restores T cell metabolic fitness in glucose-free, high fructose conditions. We find that fructose supports maximal glycolytic capacity and ATP replenishment rates in GLUT5-expressing T cells. Using steady state tracer technology, we show that 13C6 fructose supports glycolytic reprogramming and TCA anaplerosis in CAR-T cells undergoing log phase expansion. In cytotoxicity assays, GLUT5 rescues T cell cytolytic function in glucose-free medium. The fructose/GLUT5 metabolic axis also supports maximal migratory velocity, which provides mechanistic insight into why GLUT5-expressing CAR-Ts have superior effector function as they undergo "hit-and-run" serial killing. These findings translate to superior anti-tumour function in a xenograft model of AML. In fact, we found that GLUT5 enhances CAR-T cell anti-tumour function in vivo without any need for fructose intervention. Accordingly, we hypothesize that GLUT5 is sufficient to enhance CAR-T resilience by increasing the cells' competitiveness for glucose at physiologic metabolite levels. Our findings have immediate translational relevance by providing the first evidence that GLUT5 confers a competitive edge in a fructose-enriched milieu, and is a novel approach to overcome glucose depletion in hostile tumour microenvironments (TMEs).
Conflict of interest statement
Declaration of Interests ROC reports sponsored research agreements with Poseida Therapeutics outside of the submitted work.
Figures




Similar articles
-
Metabolic engineering to facilitate anti-tumor immunity.Cancer Cell. 2025 Mar 10;43(3):552-562.e9. doi: 10.1016/j.ccell.2025.02.004. Epub 2025 Feb 27. Cancer Cell. 2025. PMID: 40020672
-
Discrimination of GLUTs by Fructose Isomers Enables Simultaneous Screening of GLUT5 and GLUT2 Activity in Live Cells.ACS Chem Biol. 2023 May 19;18(5):1089-1100. doi: 10.1021/acschembio.2c00682. Epub 2023 Apr 28. ACS Chem Biol. 2023. PMID: 37116192 Free PMC article.
-
Targeting fructose metabolism by glucose transporter 5 regulation in human cholangiocarcinoma.Genes Dis. 2021 Oct 2;9(6):1727-1741. doi: 10.1016/j.gendis.2021.09.002. eCollection 2022 Nov. Genes Dis. 2021. PMID: 36157482 Free PMC article.
-
GLUT5 expression and fructose transport in human skeletal muscle.Adv Exp Med Biol. 1998;441:35-45. doi: 10.1007/978-1-4899-1928-1_4. Adv Exp Med Biol. 1998. PMID: 9781312 Review.
-
GLUT5: structure, functions, diseases and potential applications.Acta Biochim Biophys Sin (Shanghai). 2023 Oct 25;55(10):1519-1538. doi: 10.3724/abbs.2023158. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37674366 Free PMC article. Review.
References
-
- June C. H., O’Connor R. S., Kawalekar O. U., Ghassemi S. & Milone M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018). - PubMed
-
- Kawalekar O. U. et al. Distinct Signalling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity 44, 380–390 (2016). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

