This is a preprint.
A pressure-jump EPR system to monitor millisecond conformational exchange rates of spin-labeled proteins
- PMID: 38766191
- PMCID: PMC11100676
- DOI: 10.1101/2024.05.07.593074
A pressure-jump EPR system to monitor millisecond conformational exchange rates of spin-labeled proteins
Update in
-
A pressure-jump EPR system to monitor millisecond conformational exchange rates of spin-labeled proteins.Protein Sci. 2024 Dec;33(12):e5220. doi: 10.1002/pro.5220. Protein Sci. 2024. PMID: 39565088
Abstract
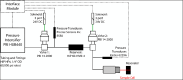
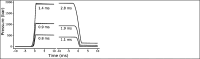
Site-directed spin labeling electron paramagnetic resonance (SDSL-EPR) using nitroxide spin labels is a well-established technology for mapping site-specific secondary and tertiary structure and for monitoring conformational changes in proteins of any degree of complexity, including membrane proteins, with high sensitivity. SDSL-EPR also provides information on protein dynamics in the time scale of ps-µs using continuous wave lineshape analysis and spin lattice relaxation time methods. However, the functionally important time domain of µs-ms, corresponding to large-scale protein motions, is inaccessible to those methods. To extend SDSL-EPR to the longer time domain, the perturbation method of pressure-jump relaxation is implemented. Here, we describe a complete high-pressure EPR system at Q-band for both static pressure and millisecond-timescale pressure-jump measurements on spin-labeled proteins. The instrument enables pressure jumps both up and down from any holding pressure, ranging from atmospheric pressure to the maximum pressure capacity of the system components (~3500 bar). To demonstrate the utility of the system, we characterize a local folding-unfolding equilibrium of T4 lysozyme. The results illustrate the ability of the system to measure thermodynamic and kinetic parameters of protein conformational exchange on the millisecond timescale.
Keywords: Electron paramagnetic resonance; conformational exchange; pressure; pressure jump; protein folding; protein kinetics; protein thermodynamics.
Conflict of interest statement
Conflict of interest statement The authors declare no conflicts of interest.
Figures




References
-
- A Vidugiris G. J., Markley J. L., & Royer C. A. (1970). Accelerated, Publications Evidence for a Molten Globule-like Transition State in Protein Folding from Determination of Activation Volumes1”. In Biochemistry. Prehoda & Markley. https://pubs.acs.org/sharingguidelines - PubMed
-
- A Vidugiris G. J., Truckses D. M., Markley J. L., & Royer C. A. (1970). High-Pressure Denaturation of Staphylococcal Nuclease Proline-to-Glycine Substitution Mutants. Ramsay & Eftink, 35, 3857–3864. - PubMed
-
- Altenbach C., & Budil D. (2023). Analyzing CW EPR Spectra of Nitroxide Labeled Macromolecules. Applied Magnetic Resonance. 10.1007/s00723-023-01610-2 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
