This is a preprint.
A conserved chronobiological complex times C. elegans development
- PMID: 38766223
- PMCID: PMC11100808
- DOI: 10.1101/2024.05.09.593322
A conserved chronobiological complex times C. elegans development
Update in
-
A conserved chronobiological complex times C. elegans development.EMBO J. 2025 Oct 20. doi: 10.1038/s44318-025-00585-z. Online ahead of print. EMBO J. 2025. PMID: 41116062
Abstract
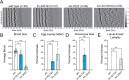
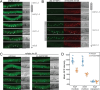
The mammalian PAS-domain protein PERIOD (PER) and its C. elegans orthologue LIN-42 have been proposed to constitute an evolutionary link between two distinct, circadian and developmental, timing systems. However, while the function of PER in animal circadian rhythms is well understood molecularly and mechanistically, this is not true for LIN-42's function in timing rhythmic development. Here, using targeted deletions, we find that the LIN-42 PAS domains are dispensable for the protein's function in timing molts. Instead, we observe arrhythmic molts upon deletion of a distinct sequence element, conserved with PER. We show that this element, designated CK1δ-binding domain (CK1BD), mediates stable binding to KIN-20, the C. elegans CK1δ/ε orthologue. We demonstrate that CK1δ phosphorylates LIN-42 and define two conserved helical motifs in the CK1BD, CK1BD-A and CK1BD-B, that have distinct roles in controlling CK1δ-binding and kinase activity in vitro. KIN-20 and the LIN-42 CK1BD are required for proper molting timing in vivo, and loss of LIN-42 binding changes KIN-20 subcellular localization. The interactions mirror the central role of a stable circadian PER-CK1 complex in setting a robust ~24-hour period. Hence, our results establish LIN-42/PER - KIN-20/CK1δ/ε as a functionally conserved signaling module of two distinct chronobiological systems.
Figures




References
-
- Gallego M., and Virshup D.M. (2007). Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol. 8, 139–148. - PubMed
-
- Gekakis N., Staknis D., Nguyen H.B., Davis F.C., Wilsbacher L.D., King D.P., Takahashi J.S., and Weitz C.J. (1998). Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569. - PubMed
-
- Lee C., Etchegaray J.P., Cagampang F.R., Loudon A.S., and Reppert S.M. (2001). Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107, 855–867. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
