This is a preprint.
Analysis of time-to-positivity data in tuberculosis treatment studies: Identifying a new limit of quantification
- PMID: 38766235
- PMCID: PMC11100935
- DOI: 10.1101/2024.05.06.24306879
Analysis of time-to-positivity data in tuberculosis treatment studies: Identifying a new limit of quantification
Update in
-
Analysis of time-to-positivity data in tuberculosis treatment studies: Identifying a new limit of quantification.Int J Antimicrob Agents. 2025 Feb;65(2):107404. doi: 10.1016/j.ijantimicag.2024.107404. Epub 2024 Dec 7. Int J Antimicrob Agents. 2025. PMID: 39653087
Abstract
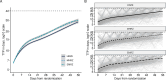
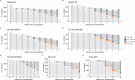
The BACTEC Mycobacteria Growth Indicator Tube (MGIT) machine is the standard globally for detecting viable mycobacteria in patients' sputum. Samples are observed for no longer than 42 days, at which point the sample is declared "negative" for tuberculosis (TB). This time to detection of bacterial growth, referred to as time-to-positivity (TTP), is increasingly of interest not solely as a diagnostic tool, but as a continuous biomarker wherein change in TTP over time can be used for comparing the bactericidal activity of different TB treatments. However, as a continuous measure, there are oddities in the distribution of TTP values observed, particularly at higher values. We explored whether there is evidence to suggest setting an upper limit of quantification (ULOQM) lower than the diagnostic limit of detection (LOD) using data from several TB-PACTS randomized clinical trials and PanACEA MAMS-TB. Across all trials, less than 7.1% of all weekly samples returned TTP measurements between 25 and 42 days. Further, the relative absolute prediction error (%) was highest in this range. When modeling with ULOQMs of 25 and 30 days, the precision in estimation improved for 23 of 25 regimen-level slopes as compared to models using the diagnostic LOD while also improving the discrimination between regimens based on Bayesian posteriors. While TTP measurements between 25 days and the diagnostic LOD may be important for diagnostic purposes, TTP values in this range may not contribute meaningfully to its use as a quantitative measure, particularly when assessing treatment response, and may lead to under-powered clinical trials.
Keywords: MGIT; clinical trials; early bactericidal activity; time-to-positivity; tuberculosis.
Figures


References
-
- Global Tuberculosis Report 2022. Geneva: World Health Organization, 2022.
-
- Chigutsa E, Patel K, Denti P, Visser M, Maartens G, Kirkpatrick CM, McIlleron H, Karlsson MO. A Time-to-Event Pharmacodynamic Model Describing Treatment Response in Patients with Pulmonary Tuberculosis Using Days to Positivity in Automated Liquid Mycobacterial Culture. Antimicrob Agents Chemother. 2013. Feb;57(2):789–95. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
