Functional dissection of parabrachial substrates in processing nociceptive information
- PMID: 38766746
- PMCID: PMC11188607
- DOI: 10.24272/j.issn.2095-8137.2023.412
Functional dissection of parabrachial substrates in processing nociceptive information
Abstract
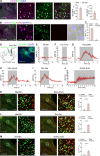
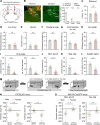
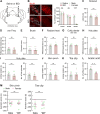
Painful stimuli elicit first-line reflexive defensive reactions and, in many cases, also evoke second-line recuperative behaviors, the latter of which reflects the sensing of tissue damage and the alleviation of suffering. The lateral parabrachial nucleus (lPBN), composed of external- (elPBN), dorsal- (dlPBN), and central/superior-subnuclei (jointly referred to as slPBN), receives sensory inputs from spinal projection neurons and plays important roles in processing affective information from external threats and body integrity disruption. However, the organizational rules of lPBN neurons that provoke diverse behaviors in response to different painful stimuli from cutaneous and deep tissues remain unclear. In this study, we used region-specific neuronal depletion or silencing approaches combined with a battery of behavioral assays to show that slPBN neurons expressing substance P receptor ( NK1R) (lPBN NK1R) are crucial for driving pain-associated self-care behaviors evoked by sustained noxious thermal and mechanical stimuli applied to skin or bone/muscle, while elPBN neurons are dispensable for driving such reactions. Notably, lPBN NK1R neurons are specifically required for forming sustained somatic pain-induced negative teaching signals and aversive memory but are not necessary for fear-learning or escape behaviors elicited by external threats. Lastly, both lPBN NK1R and elPBN neurons contribute to chemical irritant-induced nocifensive reactions. Our results reveal the functional organization of parabrachial substrates that drive distinct behavioral outcomes in response to sustained pain versus external danger under physiological conditions.
疼痛刺激往往在第一时间触发躯体的反射防御反应,并且在很多情况下,紧接着还会引起“自我抚慰疗愈”行为,后者反映了躯体对自身组织损伤的感知并寻求缓解痛苦。外侧臂旁核(lPBN)由外区(elPBN)、背区(dlPBN)和中央/上区(统称为slPBN)三个亚区组成,负责接收来自脊髓投射神经元的感觉输入,并且在处理来自躯体外部威胁和由组织受损而引起的负性情绪方面发挥了重要作用。然而,lPBN神经元是如何整合来自浅表皮肤和深层组织的不同疼痛信号,并激发多样化的行为表现仍不十分清楚。在该研究中,我们通过使用区域特异性的神经元消融或沉默手段结合一系列行为学实验,发现表达P物质受体 (NK1R) (lPBN NK1R) 的slPBN神经元对于驱动来自皮肤或骨骼/肌肉的持续性热痛和机械痛所引发的自我抚慰疗愈行为至关重要,而elPBN神经元不是驱动上述行为反应所必需。值得注意的是,lPBN NK1R 神经元对于持续性躯体痛所诱导的负性学习信号的产生,以及相关厌恶性记忆的形成是必不可少的, 而对外界危险所引起的恐惧学习或逃避反应则非必需。最后, lPBN NK1R和 elPBN神经元都介导了化学刺激物所诱导的伤害性反应。综上,我们的结果揭示了在生理条件下,持续性疼痛和外部危险刺激所引发的截然不同的行为表现是由外侧臂旁核不同亚区的神经元来驱动。.
Keywords: Defensive reaction; Lateral parabrachial nucleus; Pain affect; Somatosensory; Substance P receptor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
-
Barik A, Thompson JH, Seltzer M, et al. 2018. A brainstem-spinal circuit controlling nocifensive behavior. Neuron, 100 (6): 1491–1503. e3.
-
-
- Beecher HK The measurement of pain; prototype for the quantitative study of subjective responses. Pharmacological Reviews. 1957;9(1):59–209. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
