NEKL-4 regulates microtubule stability and mitochondrial health in ciliated neurons
- PMID: 38767515
- PMCID: PMC11104396
- DOI: 10.1083/jcb.202402006
NEKL-4 regulates microtubule stability and mitochondrial health in ciliated neurons
Abstract
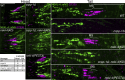
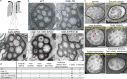
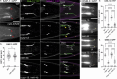
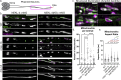
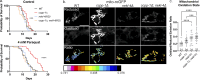
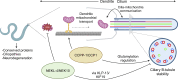
Ciliopathies are often caused by defects in the ciliary microtubule core. Glutamylation is abundant in cilia, and its dysregulation may contribute to ciliopathies and neurodegeneration. Mutation of the deglutamylase CCP1 causes infantile-onset neurodegeneration. In C. elegans, ccpp-1 loss causes age-related ciliary degradation that is suppressed by a mutation in the conserved NEK10 homolog nekl-4. NEKL-4 is absent from cilia, yet it negatively regulates ciliary stability via an unknown, glutamylation-independent mechanism. We show that NEKL-4 was mitochondria-associated. Additionally, nekl-4 mutants had longer mitochondria, a higher baseline mitochondrial oxidation state, and suppressed ccpp-1∆ mutant lifespan extension in response to oxidative stress. A kinase-dead nekl-4(KD) mutant ectopically localized to ccpp-1∆ cilia and rescued degenerating microtubule doublet B-tubules. A nondegradable nekl-4(PEST∆) mutant resembled the ccpp-1∆ mutant with dye-filling defects and B-tubule breaks. The nekl-4(PEST∆) Dyf phenotype was suppressed by mutation in the depolymerizing kinesin-8 KLP-13/KIF19A. We conclude that NEKL-4 influences ciliary stability by activating ciliary kinesins and promoting mitochondrial homeostasis.
© 2024 Power et al.
Conflict of interest statement
Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. C. Rongo reported grants from FiteBac Pharma outside the submitted work. No other disclosures were reported.
Figures





Update of
-
NEKL-4 regulates microtubule stability and mitochondrial health in C. elegans ciliated neurons.bioRxiv [Preprint]. 2024 Feb 14:2024.02.14.580304. doi: 10.1101/2024.02.14.580304. bioRxiv. 2024. Update in: J Cell Biol. 2024 Sep 2;223(9):e202402006. doi: 10.1083/jcb.202402006. PMID: 38405845 Free PMC article. Updated. Preprint.
References
-
- Alvarez Viar, G., Klena N., Martino F., Nievergelt A., and Pigino G.. 2023. The tubulin nano-code: A protofilament-specific pattern of tubulin post-translational modifications regulates ciliary beating mechanics. bioRxiv. 10.1101/2023.06.28.546853 (Preprint posted June 28, 2023). - DOI - PMC - PubMed
-
- Bae, J.-E., Jang S., Kim J.B., Hyung H., Park N.Y., Kim Y.H., Kim S.H., Kim S.H., Ha J.M., Oh G.S., et al. . 2023. Enhanced primary ciliogenesis via mitochondrial oxidative stress activates AKT to prevent neurotoxicity in HSPA9/mortalin-depleted SH-SY5Y cells. Mol. Brain. 16:41. 10.1186/s13041-023-01029-7 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

