Systemic vasculitis involving the kidney: the nephropathologist's point of view
- PMID: 38767543
- PMCID: PMC11138762
- DOI: 10.32074/1591-951X-990
Systemic vasculitis involving the kidney: the nephropathologist's point of view
Abstract
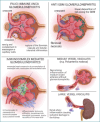
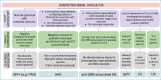
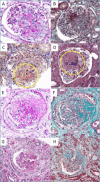
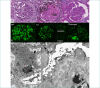
Kidneys are often targets of systemic vasculitis (SVs), being affected in many different forms and representing a possible sentinel of an underlying multi-organ condition. Renal biopsy still remains the gold standard for the identification, characterization and classification of these diseases, solving complex differential diagnosis thanks to the combined application of light microscopy (LM), immunofluorescence (IF) and electron microscopy (EM). Due to the progressively increasing complexity of renal vasculitis classification systems (e.g. pauci-immune vs immune complex related forms), a clinico-pathological approach is mandatory and adequate technical and interpretative expertise in nephropathology is required to ensure the best standard of care for our patients. In this complex background, the present review aims at summarising the current knowledge and challenges in the world of renal vasculitis, unveiling the potential role of the introduction of digital pathology in this setting, from the creation of hub-spoke networks to the future application of artificial intelligence (AI) tools to aid in the diagnostic and scoring/classification process.
Keywords: ANCA; IgA vasculitis; cryoglobulinemia; renal biopsy; systemic vasculitis.
Copyright © 2024 Società Italiana di Anatomia Patologica e Citopatologia Diagnostica, Divisione Italiana della International Academy of Pathology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis. Clin Exp Nephrol. 2013;17(5):607-10. https://doi.org/10.1007/s10157-013-0830-8 10.1007/s10157-013-0830-8 - DOI - PubMed
-
- Savage CO, Harper L, Cockwell P, et al. .ABC of arterial and vascular disease: vasculitis. BMJ. 2000;320(7245):1325-8. https://doi.org/10.1136/bmj.320.7245.1325 10.1136/bmj.320.7245.1325 - DOI - PMC - PubMed
-
- Lie JT. Illustrated histopathologic classification criteria for selected vasculitis syndromes. American College of Rheumatology Subcommittee on Classification of Vasculitis. Arthritis Rheum. 1990;33(8):1074-87. https://doi.org/10.1002/art.1780330804 10.1002/art.1780330804 - DOI - PubMed
-
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1-11. https://doi.org/10.1002/art.37715 10.1002/art.37715 - DOI - PubMed
-
- Sunderkötter CH, Zelger B, Chen KR, et al. .Nomenclature of Cutaneous Vasculitis: Dermatologic Addendum to the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol. 2018;70(2):171-84. https://doi.org/10.1002/art.40375 10.1002/art.40375 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

