Characterization of antibody response to SARS-CoV-2 Orf8 from three waves of COVID-19 outbreak in Thailand
- PMID: 38768163
- PMCID: PMC11104647
- DOI: 10.1371/journal.pone.0297272
Characterization of antibody response to SARS-CoV-2 Orf8 from three waves of COVID-19 outbreak in Thailand
Abstract
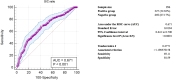
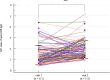
A dynamic of virus adaptation and a mass vaccination campaign could significantly reduce the severity of clinical manifestations of COVID-19 and transmission. Hence, COVID-19 may become an endemic disease globally. Moreover, mass infection as the COVID-19 pandemic progressed affected the serology of the patients as a result of virus mutation and vaccination. Therefore, a need exists to acquire accurate serological testing to monitor the emergence of new outbreaks of COVID-19 to promptly prevent and control the disease spreading. In this study, the anti-Orf8 antibodies among samples collected in Thailand's first, fourth, and fifth waves of COVID-19 outbreaks compared with pre-epidemic sera were determined by indirect ELISA. The diagnostic sensitivity and specificity of the anti-Orf8 IgG ELISA for COVID-19 samples from the first, fourth, and fifth waves of outbreaks was found to be 100% compared with pre-epidemic sera. However, the diagnostic sensitivity and specificity of the anti-Orf8 IgG ELISA for a larger number of patient samples and controls from the fifth wave of outbreaks which were collected on day 7 and 14 after an RT-PCR positive result were 58.79 and 58.44% and 89.19 and 58.44%, respectively. Our data indicated that some of the controls might have antibodies from natural past infections. Our study highlighted the potential utility of anti-Orf8 IgG antibody testing for seroprevalence surveys but still warrants further investigations.
Copyright: © 2024 Thanongsaksrikul et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- World Health Organization. Thailand: How a strong health system fights a pandemic. [cited 7 November 2023]. Available from: https://www.who.int/publications/m/item/thailand-how-a-strong-health-sys....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

