EVIDENCE meta-analysis: evaluating minimal residual disease as an intermediate clinical end point for multiple myeloma
- PMID: 38768337
- PMCID: PMC11418064
- DOI: 10.1182/blood.2024024371
EVIDENCE meta-analysis: evaluating minimal residual disease as an intermediate clinical end point for multiple myeloma
Abstract
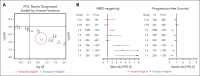
Estimating progression-free survival (PFS) and overall survival superiority during clinical trials of multiple myeloma (MM) has become increasingly challenging as novel therapeutics have improved patient outcomes. Thus, it is imperative to identify earlier end point surrogates that are predictive of long-term clinical benefit. Minimal residual disease (MRD)-negativity is a common intermediate end point that has shown prognostic value for clinical benefit in MM. This meta-analysis was based on the US Food and Drug Administration guidance for considerations for a meta-analysis of MRD as a clinical end point and evaluates MRD-negativity as an early end point reasonably likely to predict long-term clinical benefit. Eligible studies were phase 2 or 3 randomized controlled clinical trials measuring MRD-negativity as an end point in patients with MM, with follow-up of ≥6 months following an a priori-defined time point of 12 ± 3 months after randomization. Eight newly diagnosed MM studies evaluating 4907 patients were included. Trial-level associations between MRD-negativity and PFS were R2WLSiv, 0.67 (95% confidence interval [CI], 0.43-0.91) and R2copula 0.84 (0.64 to >0.99) at the 12-month time point. The individual-level association between 12-month MRD-negativity and PFS resulted in a global odds ratio (OR) of 4.02 (95% CI, 2.57-5.46). For relapse/refractory MM, there were 4 studies included, and the individual-level association between 12-month MRD-negativity and PFS resulted in a global OR of 7.67 (4.24-11.10). A clinical trial demonstrating a treatment effect on MRD is reasonably likely to eventually demonstrate a treatment effect on PFS, suggesting that MRD may be an early clinical end point reasonably likely to predict clinical benefit in MM, that may be used to support accelerated approval and thereby, expedite the availability of new drugs to patients with MM.
Conflict of interest statement
Conflict-of-interest disclosure: O.L. received research funding from National Cancer Institute (NCI)/National Institutes of Health (NIH), United States Food and Drug Administration (FDA), Leukemia & Lymphoma Society, Rising Tide Foundation, Memorial Sloan Kettering Cancer Center, Multiple Myeloma Research Foundation (MMRF), International Myeloma Foundation, Paula and Rodger Riney Foundation, Tow Foundation, Perelman Family Foundation, Myeloma Solutions Fund, Amgen, Celgene, Janssen, Takeda, Glenmark, Seattle Genetics, and Karyopharm; has received honoraria for scientific talks/participated in advisory boards for Adaptive, Amgen, Binding Site, Bristol Myers Squibb (BMS), Celgene, Cellectis, Glenmark, Janssen, Juno, and Pfizer; and served on independent data monitoring committees (IDMC) for international randomized trials by Takeda, Merck, Janssen, and Theradex. T.J.P., T.M., and C.H. are employees of Janssen Research and Development and report stock and other ownership interests in Johnson & Johnson. O.F.B. is an employee of AbbVie and reports stock and other ownership interests in AbbVie. A.B.D. is an employee of Takeda Pharmaceuticals. H.E. has a consulting or advisory role for BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GlaxoSmithKline (GSK), and Novartis, and reports receiving honoraria from BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis and travel support from BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis. H.G. received grants and/or provision of Investigational Medicinal Products from Amgen, Array Biopharma/Pfizer, BMS/Celgene, Chugai, Dietmar-Hopp-Foundation, Janssen, Johns Hopkins University, Mundipharma GmbH, Sanofi; research support from Amgen, BMS, Celgene, GlycoMimetics Inc, GSK, Heidelberg Pharma, Hoffmann-La Roche, Karyopharm, Janssen, Incyte Corporation, Millenium Pharmaceuticals Inc, Molecular Partners, Merck Sharp and Dohme, MorphoSys AG, Pfizer, Sanofi, Takeda, Novartis; serves on the advisory boards of Adaptive Biotechnology, Amgen, BMS, Janssen, Sanofi; honoraria from Amgen, BMS, Chugai, GSK, Janssen, Novartis, Sanofi, Pfizer; and received support for attending meetings and/or travel Amgen, BMS, GSK, Janssen, Novartis, Sanofi, Pfizer. S.K. has a consulting or advisory role for BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis, reports receiving honoraria from BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis, and reports travel support from BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis. C.L., I.M., C.O., and M.T. are employees of Amgen. J.A.R. is an employee of AbbVie. J.R.H. received research funding from Adaptive Biotechnologies, BioLinRx, Pfizer, Sanofi, GSK, Regeneron, Janssen, and Takeda. J.M.A. is on patient advocacy committees for Pfizer, BMS, Janssen, Takeda Oncology, and Sanofi. D.K. acknowledges research funding from NCI/NIH, FDA, MMRF, DoD-PROMETHEUS (Murtha Cancer Center Research Program), Amgen, Celgene, Janssen, and Karyopharm; has received honoraria for advisory boards and presentations for AlphaSights, Aptitude Health, Arcellx, BMS, Bridger Consulting Group, Curio Science, Karyopharm, MJH Life Sciences, MMRF, Plexus Communications, and Sanofi; and served on IDMC for Aperture Medical Technology and Arcellx. R.Z. is an employee of Sanofi. S.M.D. acknowledges research funding from Janssen and served on IDMC for Miltenyi Biotec. The remaining authors declare no competing financial interests.
Figures

Comment in
-
MRD accelerating myeloma drug development.Blood. 2024 Jul 25;144(4):345-347. doi: 10.1182/blood.2024025421. Blood. 2024. PMID: 39052265 No abstract available.
References
-
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–789. - PubMed
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

