Ketamine can produce oscillatory dynamics by engaging mechanisms dependent on the kinetics of NMDA receptors
- PMID: 38768339
- PMCID: PMC11145256
- DOI: 10.1073/pnas.2402732121
Ketamine can produce oscillatory dynamics by engaging mechanisms dependent on the kinetics of NMDA receptors
Abstract
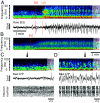
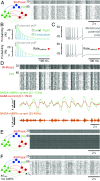
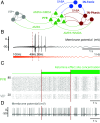
Ketamine is an N-methyl-D-aspartate (NMDA)-receptor antagonist that produces sedation, analgesia, and dissociation at low doses and profound unconsciousness with antinociception at high doses. At high and low doses, ketamine can generate gamma oscillations (>25 Hz) in the electroencephalogram (EEG). The gamma oscillations are interrupted by slow-delta oscillations (0.1 to 4 Hz) at high doses. Ketamine's primary molecular targets and its oscillatory dynamics have been characterized. However, how the actions of ketamine at the subcellular level give rise to the oscillatory dynamics observed at the network level remains unknown. By developing a biophysical model of cortical circuits, we demonstrate how NMDA-receptor antagonism by ketamine can produce the oscillatory dynamics observed in human EEG recordings and nonhuman primate local field potential recordings. We have identified how impaired NMDA-receptor kinetics can cause disinhibition in neuronal circuits and how a disinhibited interaction between NMDA-receptor-mediated excitation and GABA-receptor-mediated inhibition can produce gamma oscillations at high and low doses, and slow-delta oscillations at high doses. Our work uncovers general mechanisms for generating oscillatory brain dynamics that differs from ones previously reported and provides important insights into ketamine's mechanisms of action as an anesthetic and as a therapy for treatment-resistant depression.
Keywords: NMDA-receptor antagonism; antidepressant effect; biophysical mechanisms; gamma oscillations; slow-delta oscillations.
Conflict of interest statement
Competing interests statement:E.N.B. holds patents on anesthetic state monitoring and control. E.N.B. holds founding interest in PASCALL, a start-up developing physiological monitoring systems; receives royalties from intellectual property through Massachusetts General Hospital licensed to Masimo. The interests of E.N.B. were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies.
Figures



Update of
-
Ketamine can produce oscillatory dynamics by engaging mechanisms dependent on the kinetics of NMDA receptors.bioRxiv [Preprint]. 2024 Apr 5:2024.04.03.587998. doi: 10.1101/2024.04.03.587998. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 May 28;121(22):e2402732121. doi: 10.1073/pnas.2402732121. PMID: 38617266 Free PMC article. Updated. Preprint.
References
-
- Moghaddam B., Ketamine (MIT Press, 2021).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

