Dissecting OGT's TPR domain to identify determinants of cellular function
- PMID: 38768345
- PMCID: PMC11145291
- DOI: 10.1073/pnas.2401729121
Dissecting OGT's TPR domain to identify determinants of cellular function
Abstract
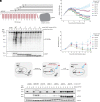
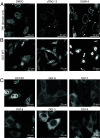
O-GlcNAc transferase (OGT) is an essential mammalian enzyme that glycosylates myriad intracellular proteins and cleaves the transcriptional coregulator Host Cell Factor 1 to regulate cell cycle processes. Via these catalytic activities as well as noncatalytic protein-protein interactions, OGT maintains cell homeostasis. OGT's tetratricopeptide repeat (TPR) domain is important in substrate recognition, but there is little information on how changing the TPR domain impacts its cellular functions. Here, we investigate how altering OGT's TPR domain impacts cell growth after the endogenous enzyme is deleted. We find that disrupting the TPR residues required for OGT dimerization leads to faster cell growth, whereas truncating the TPR domain slows cell growth. We also find that OGT requires eight of its 13 TPRs to sustain cell viability. OGT-8, like the nonviable shorter OGT variants, is mislocalized and has reduced Ser/Thr glycosylation activity; moreover, its interactions with most of wild-type OGT's binding partners are broadly attenuated. Therefore, although OGT's five N-terminal TPRs are not essential for cell viability, they are required for proper subcellular localization and for mediating many of OGT's protein-protein interactions. Because the viable OGT truncation variant we have identified preserves OGT's essential functions, it may facilitate their identification.
Keywords: O-GlcNAc transferase; OGT; TPR; cell proliferation; tetratricopeptide repeat.
Conflict of interest statement
Competing interests statement:S.J.H. has or currently serves on the SAB of Proximity Therapeutics, Psy Therapeutics, Frequency Therapeutics, Souvien Therapeutics, Sensorium Therapeutics, 4M Therapeutics, Ilios Therapeutics, Entheos Labs, and the Kissick Family Foundation FTD Grant Program, none of whom were involved in the present study. S.J.H. has also received speaking or consulting fees from Amgen, AstraZeneca, Biogen, Merck, Regenacy Pharmaceuticals, Syros Pharmaceuticals, Juvenescence Life, Neumora Therapeutics, and Biohaven Pharmaceuticals, as well as sponsored research or gift funding from AstraZeneca, JW Pharmaceuticals, Lexicon Pharmaceuticals, Vesigen Therapeutics, Compass Pathways, Atai Life Sciences, and Stealth Biotherapeutics. The funders had no role in the design or content of this article, or the decision to submit this review for publication. S.C.P., B.E.G., F.A.H., C.M.J., J.A.P., J.J, Z.G.L., G.Q.F., and S.W. declare no competing interests.
Figures




References
-
- Zachara N. E., et al. , Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J. Biol. Chem. 279, 30133–30142 (2004). - PubMed
-
- Zhang F., et al. , O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell 115, 715–725 (2003). - PubMed
MeSH terms
Substances
Grants and funding
- P50 GM107618/GM/NIGMS NIH HHS/United States
- F32 GM129889/GM/NIGMS NIH HHS/United States
- R01 GM094263-11S1/HHS | National Institutes of Health (NIH)
- PGS-M PGS-D3/Canadian Government | Natural Sciences and Engineering Research Council of Canada (NSERC)
- Stuart and Suzanne Steele MGH Research Scholar Award/Massachusetts General Hospital (MGH)
LinkOut - more resources
Full Text Sources
Miscellaneous

