Molecular Pathways and Cellular Subsets Associated with Adverse Clinical Outcomes in Overlapping Immune-Related Myocarditis and Myositis
- PMID: 38768394
- PMCID: PMC12302522
- DOI: 10.1158/2326-6066.CIR-24-0011
Molecular Pathways and Cellular Subsets Associated with Adverse Clinical Outcomes in Overlapping Immune-Related Myocarditis and Myositis
Abstract
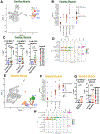
Immune checkpoint therapies (ICT) can induce life-threatening immune-related adverse events, including myocarditis and myositis, which are rare but often concurrent. The molecular pathways and immune subsets underlying these toxicities remain poorly understood. To address this need, we performed single-cell RNA sequencing of heart and skeletal muscle biopsies obtained from living patients with cancers treated with ICTs and admitted to the hospital with myocarditis and/or myositis (overlapping myocarditis plus myositis, n = 10; myocarditis-only, n = 1) or ICT-exposed patients ruled out for toxicity utilized as controls (n = 9). All biopsies were obtained within 96 hours of clinical presentation. Analyses of 58,523 cells revealed CD8+ T cells with a cytotoxic phenotype expressing activation/exhaustion markers in both myocarditis and myositis. Furthermore, the analyses identified a population of myeloid cells expressing tissue-resident signatures and FcγRIIIa (CD16a), which is known to bind IgG and regulate complement activation. Immunohistochemistry of affected cardiac and skeletal muscle tissues revealed protein expression of pan-IgG and complement product C4d, which were associated with the presence of high-titer serum autoantibodies against muscle antigens in a subset of patients. We further identified a population of inflammatory IL1B+TNF+ myeloid cells specifically enriched in myocarditis and associated with greater toxicity severity and poorer clinical outcomes. These results provide insight into the myeloid subsets present in human immune-related myocarditis and myositis tissues and nominate new targets for investigation into rational treatments to overcome these high-mortality toxicities. See related Spotlight by Fankhauser et al., p. 954.
©2024 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest Statement:
S.B., Z.H., M.B.B., C.I., A.D., A.R-K., S.S.Y., and R.A.S. report no competing interests. B.A.S. reports support from an American Society of Clinical Oncology (ASCO) Conquer Cancer Foundation (CCF) Career Development Award, and Consulting/Advisory Role: Merck. N.L.P. reports support by Cancer Prevention & Research Institute of Texas Early Clinical Investigator Award, NIH/NCI, and the Andrew Sabin Family Foundation; consulting relationships with Replimmune, Kiniksa, and honoraria from Physician Education Resource, Society for Immunotherapy for Cancer, and Scripps. M.B reports honorarium from Elsevier and Springer. J.P.A is a Scientific Advisory Committee Member for Achelois, Affini-T, Apricity, Asher Bio, BioAtla LLC, Candel Therapeutics, Catalio, Carisma, Codiak Biosciences, Inc, C-Reveal Therapueutics, Dragonfly Therapeutics, Earli Inc, Enable Medicine, Glympse, Henlius/Hengenix, Hummingbird, ImaginAb, Infinity Pharma, InterVenn Biosciences, LAVA Therapeuticss, Oncolytics, PBM Capital, Phenomic AI, Time Bioventures, Two Bear Capital, and Xilis,Inc. He has private investments with Adaptive Biotechnologies, BioNTech, JSL Health, Sporos, and Time Bioventures. P.S is a Scientific Advisory Committee Member for Achelois, Adaptive Biotechnologies, Affini-T, Apricity, Asher Bio, BioAtla LLC, Candel Therapeutics, Catalio, Carisma, Codiak Biosciences, Inc, C-Reveal Therapueutics, Dragonfly Thereapeutics, Earli Inc, Enable Medicine, Glympse, Henlius/Hengenix, ImaginAb, Infinity Pharma, InterVenn Biosciences, JSL Health, LAVA Therapeuticss, Oncolytics, PBM Capital, Phenomic AI, Time Bioventures, Two Bear Capital, and Xilis,Inc. She has private investments with BioNTech, JSL Health, Sporos, and Time Bioventures. S.K.S. reports Consulting or Advisory Role: Amgen, Apricity Health LLC, Arcus Biosciences, Baird, Bayer, Boxer Capital, Breaking Data, Bristol-Myers Squibb, Cancer Expert Now, ChemoCentryx, Dendreon, InProTher, Johnson & Johnson, Javelin Oncology, Kahr Medical Ltd, Macrogenics, MD Education Limited, Merck, OncLive (Owned by Intellisphere, LLC), Pfizer, Portage, Regeneron, Rondo Theraputics and The Clinical Comms Group; Research: AstraZeneca, Johnson & Johnson, and Regeneron.
Figures






References
-
- Zhou Y, Medik YB, Patel B, Zamler DB, Chen S, Chapman T, et al. Intestinal toxicity to CTLA-4 blockade driven by IL-6 and myeloid infiltration. J Exp Med [Internet]. 2023;220. Available from: http://www.ncbi.nlm.nih.gov/pubmed/36367776 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

