Antibody-displaying extracellular vesicles for targeted cancer therapy
- PMID: 38769158
- PMCID: PMC11584392
- DOI: 10.1038/s41551-024-01214-6
Antibody-displaying extracellular vesicles for targeted cancer therapy
Abstract
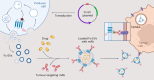
Extracellular vesicles (EVs) function as natural delivery vectors and mediators of biological signals across tissues. Here, by leveraging these functionalities, we show that EVs decorated with an antibody-binding moiety specific for the fragment crystallizable (Fc) domain can be used as a modular delivery system for targeted cancer therapy. The Fc-EVs can be decorated with different types of immunoglobulin G antibody and thus be targeted to virtually any tissue of interest. Following optimization of the engineered EVs by screening Fc-binding and EV-sorting moieties, we show the targeting of EVs to cancer cells displaying the human epidermal receptor 2 or the programmed-death ligand 1, as well as lower tumour burden and extended survival of mice with subcutaneous melanoma tumours when systemically injected with EVs displaying an antibody for the programmed-death ligand 1 and loaded with the chemotherapeutic doxorubicin. EVs with Fc-binding domains may be adapted to display other Fc-fused proteins, bispecific antibodies and antibody-drug conjugates.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: S.E.A. is a founder of and a consultant for Evox Therapeutics. D.G., J.Z.N. and A.G. are consultants for Evox Therapeutics. O.P.B.W., D.G., J.Z.N., A.G. and S.E.A. have stock interest in Evox Therapeutics. H.S. and A.L. are employees of Evox Therapeutics. The other authors declare no competing interests.
Figures






References
-
- Borcoman, E. et al. Novel patterns of response under immunotherapy. Ann. Oncol.30, 385–396 (2019). - PubMed
-
- Kooijmans, S. A. A., de Jong, O. G. & Schiffelers, R. M. Exploring interactions between extracellular vesicles and cells for innovative drug delivery system design. Adv. Drug Deliv. Rev.173, 252–278 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

