Pathogen-epithelium interactions and inflammatory responses in Salmonella Dublin infections using ileal monolayer models derived from adult bovine organoids
- PMID: 38769412
- PMCID: PMC11106274
- DOI: 10.1038/s41598-024-62407-2
Pathogen-epithelium interactions and inflammatory responses in Salmonella Dublin infections using ileal monolayer models derived from adult bovine organoids
Abstract
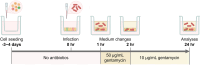
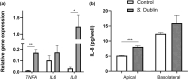
Salmonella enterica serovar Dublin (S. Dublin) is an important enteric pathogen affecting cattle and poses increasing public health risks. Understanding the pathophysiology and host-pathogen interactions of S. Dublin infection are critical for developing effective control strategies, yet studies are hindered by the lack of physiologically relevant in vitro models. This study aimed to generate a robust ileal monolayer derived from adult bovine organoids, validate its feasibility as an in vitro infection model with S. Dublin, and evaluate the epithelial response to infection. A stable, confluent monolayer with a functional epithelial barrier was established under optimized culture conditions. The model's applicability for studying S. Dublin infection was confirmed by documenting intracellular bacterial invasion and replication, impacts on epithelial integrity, and a specific inflammatory response, providing insights into the pathogen-epithelium interactions. The study underscores the utility of organoid-derived monolayers in advancing our understanding of enteric infections in livestock and highlights implications for therapeutic strategy development and preventive measures, with potential applications extending to both veterinary and human medicine. The established bovine ileal monolayer offers a novel and physiologically relevant in vitro platform for investigating enteric pathogen-host interactions, particularly for pathogens like S. Dublin.
Keywords: S. Dublin; Salmonella; Adult stem cells; Bovine ileum; In vitro infection model; Organoid-derived monolayer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

