Adipocyte HSL is required for maintaining circulating vitamin A and RBP4 levels during fasting
- PMID: 38769419
- PMCID: PMC11239848
- DOI: 10.1038/s44319-024-00158-x
Adipocyte HSL is required for maintaining circulating vitamin A and RBP4 levels during fasting
Abstract
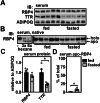
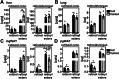
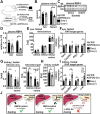
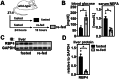
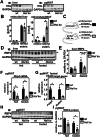
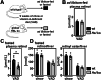
Vitamin A (retinol) is distributed via the blood bound to its specific carrier protein, retinol-binding protein 4 (RBP4). Retinol-loaded RBP4 is secreted into the circulation exclusively from hepatocytes, thereby mobilizing hepatic retinoid stores that represent the major vitamin A reserves in the body. The relevance of extrahepatic retinoid stores for circulating retinol and RBP4 levels that are usually kept within narrow physiological limits is unknown. Here, we show that fasting affects retinoid mobilization in a tissue-specific manner, and that hormone-sensitive lipase (HSL) in adipose tissue is required to maintain serum concentrations of retinol and RBP4 during fasting in mice. We found that extracellular retinol-free apo-RBP4 induces retinol release by adipocytes in an HSL-dependent manner. Consistently, global or adipocyte-specific HSL deficiency leads to an accumulation of retinoids in adipose tissue and a drop of serum retinol and RBP4 during fasting, which affects retinoid-responsive gene expression in eye and kidney and lowers renal retinoid content. These findings establish a novel crosstalk between liver and adipose tissue retinoid stores for the maintenance of systemic vitamin A homeostasis during fasting.
Keywords: Fasting; HSL; RBP4; Retinol; Vitamin A.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

