Non-invasive spinal cord electrical stimulation for arm and hand function in chronic tetraplegia: a safety and efficacy trial
- PMID: 38769431
- PMCID: PMC11108781
- DOI: 10.1038/s41591-024-02940-9
Non-invasive spinal cord electrical stimulation for arm and hand function in chronic tetraplegia: a safety and efficacy trial
Abstract
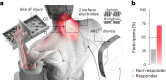
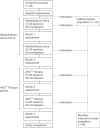
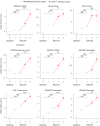
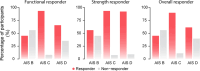
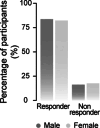
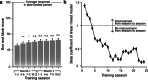
Cervical spinal cord injury (SCI) leads to permanent impairment of arm and hand functions. Here we conducted a prospective, single-arm, multicenter, open-label, non-significant risk trial that evaluated the safety and efficacy of ARCEX Therapy to improve arm and hand functions in people with chronic SCI. ARCEX Therapy involves the delivery of externally applied electrical stimulation over the cervical spinal cord during structured rehabilitation. The primary endpoints were safety and efficacy as measured by whether the majority of participants exhibited significant improvement in both strength and functional performance in response to ARCEX Therapy compared to the end of an equivalent period of rehabilitation alone. Sixty participants completed the protocol. No serious adverse events related to ARCEX Therapy were reported, and the primary effectiveness endpoint was met. Seventy-two percent of participants demonstrated improvements greater than the minimally important difference criteria for both strength and functional domains. Secondary endpoint analysis revealed significant improvements in fingertip pinch force, hand prehension and strength, upper extremity motor and sensory abilities and self-reported increases in quality of life. These results demonstrate the safety and efficacy of ARCEX Therapy to improve hand and arm functions in people living with cervical SCI. ClinicalTrials.gov identifier: NCT04697472 .
© 2024. The Author(s).
Conflict of interest statement
The Up-LIFT study investigators hold various patents in relation with the present work, act as consultants to ONWARD Medical and may be minority shareholders of ONWARD Medical.
Figures
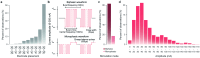


References
-
- Anderson, M. A. et al. Natural and targeted circuit reorganization after spinal cord injury. Nat. Neurosci. 25, 1584–1596 (2022). - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

