Therapies for cutaneous squamous cell carcinoma in recessive dystrophic epidermolysis bullosa: a systematic review of 157 cases
- PMID: 38769503
- PMCID: PMC11106883
- DOI: 10.1186/s13023-024-03190-1
Therapies for cutaneous squamous cell carcinoma in recessive dystrophic epidermolysis bullosa: a systematic review of 157 cases
Abstract
Background: Invasive cutaneous squamous cell carcinomas (cSCC) are a leading cause of death in recessive dystrophic epidermolysis bullosa (RDEB), a rare blistering genodermatosis. Outcomes of RDEB-cSCC therapies have primarily been described in case reports. Systematic studies are scarce. This systematic review aims to assess the pathophysiology, clinical characteristics, and outcomes of RDEB-cSCCs, with a focus on results and mechanisms of recent immunotherapies and anti-EGFR treatments.
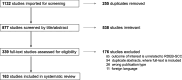
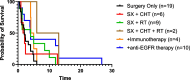
Results: A systematic literature search of epidermolysis bullosa and cSCC was performed in February 2024, using PubMed, Embase, Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, and EudraCT databases. Cases with administration of systematic therapies and unpublished outcomes regarding death were tracked with corresponding authors. Data extraction and risk of bias assessment was performed by two independent reviewers. Of 1132 references in the original search, 163 relevant articles were identified, representing 59 case reports, 7 cohort studies, 49 abstracts, 47 in-vitro/in-vivo experiments, and 1 bioinformatic study. From these, 157 cases of RDEB-cSCCs were included. The majority of RDEB-cSCCs were well-differentiated (64.1%), ulcerated (59.6%), and at least 2 cm in size (77.6%), with a median age at diagnosis of 30 years old (range 6-68.4). Surgery was the primary form of treatment (n = 128), followed by chemotherapy and radiotherapy. Anti-EGFR therapy and immunotherapy was also reported beginning in 2009 and 2019, respectively. Survival time from first cSCC diagnosis to death was available in 50 cases. When stratified by their treatment regimen, median survival time was 1.85 years (surgery + chemotherapy, n = 6), 2 years (surgery only, n = 19), 4.0 years (+ anti-EFGR therapy, n = 10), 4 years (surgery + radiotherapy, n = 9), 4.6 years (+ immunotherapy, n = 4), and 9.5 years (surgery + chemotherapy + radiotherapy; n = 2). Treatment-related adverse events were primarily limited to impaired wound healing for immunotherapies and nausea and fatigue for anti-EGFR therapies.
Conclusions: Despite the challenges of a limited sample size in a rare disease, this systematic review provides an overview of treatment options for cSCCs in RDEB. When surgical treatment options have been exhausted, the addition of immunotherapy and/or anti-EGFR therapies may extend patient survival. However, it is difficult to attribute extended survival to any single treatment, as multiple therapeutic modalities are often used to treat RDEB-cSCCs.
Keywords: Anti-EGFR therapy; Chemotherapy; Epidermolysis bullosa; Immunotherapy; Patient survival; Radiotherapy; Recessive dystrophic epidermolysis bullosa; Skin cancer; Squamous cell carcinoma; Therapy; Treatment.
© 2024. The Author(s).
Conflict of interest statement
ASP is an Investigator for AbbVie, Applied Pharma Research, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, Regeneron, UCB; Consultant for Aegerion Pharma, Azitra, BioCryst, Boehringer-Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen, Johnson & Johnson, Krystal, LEO Pharma, Novartis, Primus, Regeneron, Sanofi/Genzyme, Seanergy, TWI Biotechnology, UCB; and Data Safety Monitoring Board for AbbVie, Abeona, Catawba, Galderma, InMed.
None of the other authors report conflicts of interest.
Figures
References
-
- Cho RJ, Alexandrov LB, den Breems NY, Atanasova VS, Farshchian M, Purdom E, et al. APOBEC mutation drives early-onset squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2018; 10.1126/scitranslmed.aas9668. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

