Bio-nanoparticles loaded with synovial-derived exosomes ameliorate osteoarthritis progression by modifying the oxidative microenvironment
- PMID: 38769545
- PMCID: PMC11103857
- DOI: 10.1186/s12951-024-02538-w
Bio-nanoparticles loaded with synovial-derived exosomes ameliorate osteoarthritis progression by modifying the oxidative microenvironment
Abstract
Background and aims: Osteoarthritis (OA) is a prevalent degenerative joint disorder, marked by the progressive degeneration of joint cartilage, synovial inflammation, and subchondral bone hyperplasia. The synovial tissue plays a pivotal role in cartilage regulation. Exosomes (EXOs), small membrane-bound vesicles released by cells into the extracellular space, are crucial in mediating intercellular communication and facilitating the exchange of information between tissues. Our study aimed to devise a hydrogel microsphere infused with SOD3-enriched exosomes (S-EXOs) to protect cartilage and introduce a novel, effective approach for OA treatment.
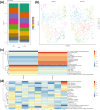
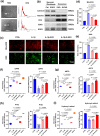
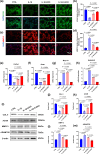
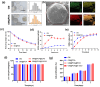
Materials and methods: We analyzed single-cell sequencing data from 4247 cells obtained from the GEO database. Techniques such as PCR, Western Blot, immunofluorescence (IF), and assays to measure oxidative stress levels were employed to validate the cartilage-protective properties of the identified key protein, SOD3. In vivo, OA mice received intra-articular injections of S-EXOs bearing hydrogel microspheres, and the effectiveness was assessed using safranine O (S.O) staining and IF.
Results: Single-cell sequencing data analysis suggested that the synovium influences cartilage via the exocrine release of SOD3. Our findings revealed that purified S-EXOs enhanced antioxidant capacity of chondrocytes, and maintained extracellular matrix metabolism stability. The S-EXO group showed a significant reduction in mitoROS and ROS levels by 164.2% (P < 0.0001) and 142.7% (P < 0.0001), respectively, compared to the IL-1β group. Furthermore, the S-EXO group exhibited increased COL II and ACAN levels, with increments of 2.1-fold (P < 0.0001) and 3.1-fold (P < 0.0001), respectively, over the IL-1β group. Additionally, the S-EXO group showed a decrease in MMP13 and ADAMTS5 protein expression by 42.3% (P < 0.0001) and 44.4% (P < 0.0001), respectively. It was found that S-EXO-containing hydrogel microspheres could effectively deliver SOD3 to cartilage and significantly mitigate OA progression. The OARSI score in the S-EXO microsphere group markedly decreased (P < 0.0001) compared to the OA group.
Conclusion: The study demonstrated that the S-EXOs secreted by synovial fibroblasts exert a protective effect on chondrocytes, and microspheres laden with S-EXOs offer a promising therapeutic alternative for OA treatment.
Keywords: Exosomes; Microspheres; Osteoarthritis; SOD3.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

