Galectin-receptor interaction: a key player in liver fibrosis induced by Schistosoma japonicum infection
- PMID: 38769548
- PMCID: PMC11106894
- DOI: 10.1186/s13071-024-06314-5
Galectin-receptor interaction: a key player in liver fibrosis induced by Schistosoma japonicum infection
Abstract
Background: Schistosoma japonicum eggs lodge in the liver and induce a fibrotic granulomatous immune response in the liver of host. Galectin 3 (Gal-3) is a protein implicated in fibrosis in multiple organs. However, the pathology and molecular mechanisms promoting hepatic granuloma formation remain poorly understood.
Methods: To investigate the effect of blocking galectin-receptor interactions by α-lactose on liver immunopathology in mice with S. japonicum infection, C57BL/6 mice were infected with S. japonicum and alpha (α)-lactose was intraperitoneally injected to block the interactions of galectins and their receptors.
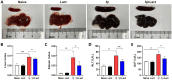
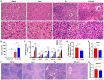
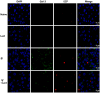
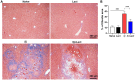
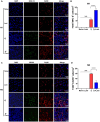
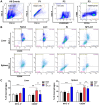
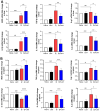
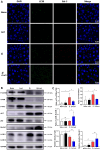
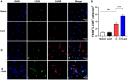
Results: Compared with S. japonicum-infected mice, there were significantly decreased Gal-3 mRNA and protein expression levels, decreased intensity of Gal-3 fluorescence in the liver, decreased serum ALT and AST levels, decreased egg numbers of S. japonicum in the liver section, attenuated hepatic and spleen pathology, and alleviated liver fibrosis accompanied with decreased protein expression levels of fibrosis markers [α-smooth muscle actin (α-SMA), collagen I, and collagen IV] in the liver of S. japonicum-infected mice blocked galectin-receptor interactions with hematoxylin-eosin staining, Masson's trichrome staining, immunohistochemistry, or Western blot analysis. Compared with S. japonicum-infected mice, blocking galectin-receptor interactions led to increased eosinophil infiltration and higher eosinophil cationic protein (ECP) expression in the liver, accompanied by increased mRNA levels of eosinophil granule proteins [ECP and eosinophil peroxidase (EPO)], IL-5, CCL11, and CCR3 in the liver and decreased mRNA levels of Gal-3 and M2 macrophage cytokines (TGF-β, IL-10, and IL-4) in the liver and spleen by using quantitative real-time reverse transcription-polymerase chain reaction. In addition, there were increased Beclin1 protein expression and protein expression ratio of LC3B-II/LC3B-I and decreased p62 protein expression and protein expression ratios of phospho-mTOR/mTOR and phospho-AKT/AKT by Western blot; increased double-labeled F4/80+/LC3B+ cells by immunofluorescence staining; increased M1 macrophage polarization in the liver of S. japonicum-infected mice blocked galectin-receptor interactions by flow cytometric analysis and immunofluorescence staining.
Conclusions: Our data found that blockage of galectin-receptor interactions downregulated Gal-3, which in turn led to reduced liver functional damage, elevated liver eosinophil recruitment, promoted macrophage autophagy through the Akt/mTOR signaling pathway, and alleviated liver pathology and fibrosis. Therefore, Gal-3 plays a pivotal role during S. japonicum infection and could be a target of pharmacologic potential for liver fibrosis induced by S. japonicum infection.
Keywords: Schistosoma japonicum; Galectin-receptor interactions; Liver fibrosis; Macrophage autophagy; Mice.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- World Health Organization. Schistosomiasis (Bilharzia). https://www.who.int/health-topics/schistosomiasis#tab=tab_1
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

