Scn1a haploinsufficiency in the prefrontal cortex leads to cognitive impairment and depressive phenotype
- PMID: 38769595
- PMCID: PMC11729715
- DOI: 10.1093/brain/awae167
Scn1a haploinsufficiency in the prefrontal cortex leads to cognitive impairment and depressive phenotype
Abstract
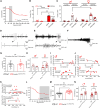
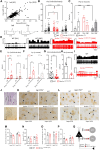
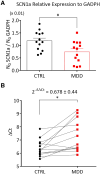
Altered development and function of the prefrontal cortex (PFC) during adolescence is implicated in the origin of mental disorders. Deficits in the GABAergic system prominently contribute to these alterations. Nav1.1 is a voltage-gated Na+ channel critical for normal GABAergic activity. Here, we studied the role of Nav1.1 in PFC function and its potential relationship with the aetiology of mental disorders. Dysfunction of Nav1.1 activity in the medial PFC (mPFC) of adolescent mice enhanced the local excitation/inhibition ratio, resulting in epileptic activity, cognitive deficits and depressive-like behaviour in adulthood, along with a gene expression profile linked to major depressive disorder (MDD). Additionally, it reduced extracellular serotonin concentration in the dorsal raphe nucleus and brain-derived neurotrophic factor expression in the hippocampus, two MDD-related brain areas beyond the PFC. We also observed alterations in oscillatory activity and impaired hippocampal-mPFC coherence during sleep. Finally, we found reduced expression levels of SCN1A, the gene encoding Nav1.1, in post-mortem PFC samples from human MDD subjects. Collectively, our results provide a novel mechanistic framework linking adolescence-specific alterations in Nav1.1 function in the PFC to the pathogenesis of epilepsy and comorbidities such as cognitive impairment and depressive disorders.
Keywords: GABAergic interneurons; Nav1.1 channel; excitatory/inhibitory balance (E/I balance); mayor depressive disorder; prefrontal cortex.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. - PubMed
MeSH terms
Substances
Grants and funding
- 202207/Catalan Government
- CB/07/09/0034/CIBERSAM
- US-1264375/Programa Operativo FEDER Andalucía 2014-2020
- PAIUJA-EI_CTS02_2023/Universidad de Jaén
- BIO-302/Junta de Andalucía
- OTR08262-2021/Fundación Alicia Koplowitz
- Asociación Síndrome STXBP1
- IT211/19/Basque Government
- Ministry of Health
- RH-0048-2020/Consumer of the Andalusian Government
LinkOut - more resources
Full Text Sources
Miscellaneous

