Item performance of the scale for the assessment and rating of ataxia in rare and ultra-rare genetic ataxias
- PMID: 38769902
- PMCID: PMC11330187
- DOI: 10.1002/psp4.13162
Item performance of the scale for the assessment and rating of ataxia in rare and ultra-rare genetic ataxias
Abstract
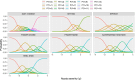
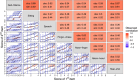
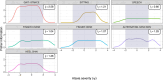
The Scale for the Assessment and Rating of Ataxia (SARA) is widely used for assessing the severity and progression of genetic cerebellar ataxias. SARA is now considered a primary end point in several ataxia treatment trials, but its underlying composite item measurement model has not yet been tested. This work aimed to evaluate the composite properties of SARA and its items using item response theory (IRT) and to demonstrate its applicability across even ultra-rare genetic ataxias. Leveraging SARA subscores data from 1932 visits from 990 patients of the Autosomal Recessive Cerebellar Ataxias (ARCA) registry, we assessed the performance of SARA using IRT methodology. The item characteristics were evaluated over the ataxia severity range of the entire ataxia population as well as the assessment validity across 115 genetic ARCA subpopulations. A unidimensional IRT model was able to describe SARA item data, indicating that SARA captures one single latent variable. All items had high discrimination values (1.5-2.9) indicating the effectiveness of the SARA in differentiating between subjects with different disease statuses. Each item contributed between 7% and 28% of the total assessment informativeness. There was no evidence for differences between the 115 genetic ARCA subpopulations in SARA applicability. These results show the good discrimination ability of SARA with all of its items adding informational value. The IRT framework provides a thorough description of SARA on the item level, and facilitates its utilization as a clinical outcome assessment in upcoming longitudinal natural history or treatment trials, across a large number of ataxias, including ultra-rare ones.
© 2024 The Author(s). CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Dr. Klockgether is receiving research support from the Bundesministerium für Bildung und Forschung (BMBF), the National Institutes of Health (NIH), and Servier. Within the last 24 months, he has received consulting fees from Biogen, UCB, and Vico Therapeutics, all unrelated to the present manuscript. Dr. Synofzik has received consultancy honoraria from Ionis, UCB, Prevail, Orphazyme, Servier, Reata, GenOrph, AviadoBio, Biohaven, Zevra, and Lilly, all unrelated to the present manuscript. Drs Hooker and Karlsson have received consultancy fees from and own stock in Pharmetheus, all unrelated to this manuscript. As an Associate Editor
Figures


References
-
- Anheim M, Tranchant C, Koenig M. The autosomal recessive cerebellar ataxias. N Engl J Med. 2012;366(7):636‐646. - PubMed
-
- Synofzik M, Puccio H, Mochel F, Schöls L. Autosomal recessive cerebellar ataxias: paving the way toward targeted molecular therapies. Neuron. 2019;101(4):560‐583. - PubMed
-
- Klockgether T, Ashizawa T, Brais B, et al. Paving the way toward meaningful trials in ataxias: an ataxia global initiative perspective. Mov Disord. 2022;37(6):1125‐1130. - PubMed
-
- Schmitz‐Hübsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717‐1720. - PubMed
-
- Perez‐Lloret S, van de Warrenburg B, Rossi M, et al. Assessment of ataxia rating scales and cerebellar functional tests: critique and recommendations. Mov Disord. 2021;36(2):283‐297. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

