Characteristics and outcomes of gemcitabine-associated pulmonary hypertension
- PMID: 38770007
- PMCID: PMC11103709
- DOI: 10.1183/23120541.00654-2023
Characteristics and outcomes of gemcitabine-associated pulmonary hypertension
Abstract
Background: Despite its known cardiac and lung toxicities, the chemotherapy drug gemcitabine has only rarely been associated with pulmonary hypertension (PH), and the underlying mechanism remains unclear. The objective of the present study was to assess the association between gemcitabine and PH.
Methods: We identified incident cases of precapillary PH confirmed by right heart catheterisation in patients treated with gemcitabine from the French PH Registry between January 2007 and December 2022. The aetiology, clinical, functional, radiological and haemodynamic characteristics of PH were reviewed at baseline and during follow-up. A pharmacovigilance disproportionality analysis was conducted using the World Health Organization (WHO) pharmacovigilance database.
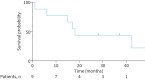
Results: We identified nine cases of pulmonary arterial hypertension, either induced (in eight patients) or exacerbated (in one patient) by gemcitabine. Patients exhibited severe precapillary PH, with a median mean pulmonary arterial pressure of 40 (range 26-47) mmHg, a cardiac index of 2.4 (1.6-3.9) L·min-1·m-2 and a pulmonary vascular resistance of 6.3 (3.1-12.6) Wood units. The median time from the initiation of gemcitabine to the onset of PH was 7 (4-50) months, with patients receiving a median of 16 (6-24) gemcitabine injections. Six patients showed clinical improvement upon discontinuation of gemcitabine. In the WHO pharmacovigilance database, we identified a significant signal with 109 cases reporting at least one adverse event related to PH with gemcitabine.
Conclusion: Both clinical cases and pharmacovigilance data substantiate a significant association between gemcitabine use and the onset or worsening of precapillary PH. The observed improvement following the discontinuation of treatment underscores the importance of PH screening in gemcitabine-exposed patients experiencing unexplained dyspnoea.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: X. Jais reports grants or contracts from Acceleron, Janssen, MSD and Bayer HealthCare, outside the submitted work; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Janssen and MSD, outside the submitted work. Conflict of interest: L. Savale reports grants or contracts from Bayer, Janssen and Merck, outside the submitted work; and speaker fees from Janssen, outside the submitted work. Conflict of interest: O. Sitbon reports grants or contracts from Acceleron, AOP Orphan, Janssen, GSK and MSD, outside the submitted work; consulting fees from Altavant, Gossamer Bio, Janssen and MSD, outside the submitted work; payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events for AOP Orphan, Janssen, Ferrer and MSD, outside the submitted work; and participation on a Data Safety Monitoring Board or Advisory Board for Acceleron, Altavant, Gossamer Bio, Janssen, MSD and Ferrer, outside the submitted work. Conflict of interest: M. Humbert reports grants or contracts from Acceleron, AOP Orphan, Janssen, Merck and Shou Ti, outside the submitted work; consulting fees from Acceleron, Aerovate, Altavant, AOP Orphan, Bayer, Chiesi, Ferrer, Janssen, Merck, MorphogenIX, Shou Ti, Tiakis and United Therapeutics, outside the submitted work; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events for Janssen and Merck, outside the submitted work; and participation on a Data Safety Monitoring Board or Advisory Board for Acceleron, Altavant, Janssen, Merck and United Therapeutics, outside the submitted work. Conflict of interest: P. Bonniaud reports a research grant from AstraZeneca, outside the submitted work; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Sanofi and AstraZeneca, outside the submitted work; support for attending medical and research meetings from AstraZeneca, Novartis, Sanofi, Roche and Boehringer, outside the submitted work; and personal fees for advisory boards from AstraZeneca, Novartis, Sanofi, GSK, Roche and Boehringer, outside the submitted work. Conflict of interest: D. Montani reports grants or contracts from Acceleron, Janssen and Merck MSD, outside the submitted work; consulting fees from Acceleron, Merck MSD, Janssen and Ferrer, outside the submitted work; and speaker fees from Bayer, Janssen, Boehringer, Chiesi, GSK, Ferrer and Merck MSD, outside the submitted work. Conflict of interest: The remaining authors have nothing to disclose.
Figures
Similar articles
-
Association between Leflunomide and Pulmonary Hypertension.Ann Am Thorac Soc. 2021 Aug;18(8):1306-1315. doi: 10.1513/AnnalsATS.202008-913OC. Ann Am Thorac Soc. 2021. PMID: 33502958
-
Association of Pulmonary Hypertension With Trastuzumab Emtansine: An Analysis of French Pulmonary Hypertension Registry and WHO Pharmacovigilance Database.Chest. 2025 May;167(5):1468-1480. doi: 10.1016/j.chest.2024.11.006. Epub 2024 Nov 19. Chest. 2025. PMID: 39571726
-
Characteristics and outcomes of patients developing pulmonary hypertension associated with proteasome inhibitors.Eur Respir J. 2024 Jun 28;63(6):2302158. doi: 10.1183/13993003.02158-2023. Print 2024 Jun. Eur Respir J. 2024. PMID: 38697649
-
Characteristics and Long-term Outcomes of Pulmonary Venoocclusive Disease Induced by Mitomycin C.Chest. 2021 Mar;159(3):1197-1207. doi: 10.1016/j.chest.2020.09.238. Epub 2020 Sep 24. Chest. 2021. PMID: 32979348
-
Phosphodiesterase 5 inhibitors for pulmonary hypertension.Cochrane Database Syst Rev. 2019 Jan 31;1(1):CD012621. doi: 10.1002/14651858.CD012621.pub2. Cochrane Database Syst Rev. 2019. PMID: 30701543 Free PMC article.
References
-
- Shepherd FA, Cormier Y, Burkes R, et al. . Phase II trial of gemcitabine and weekly cisplatin for advanced non-small cell lung cancer. Semin Oncol 1997; 24: 3 Suppl. 8, S8-27–S8-30. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
