In-Vitro Cytotoxicity, Apoptotic Property, and Gene Expression Changes Induced by Naringenin-7-O-Glucoside in Triple-Negative Breast Cancer
- PMID: 38770462
- PMCID: PMC11104259
- DOI: 10.7759/cureus.58634
In-Vitro Cytotoxicity, Apoptotic Property, and Gene Expression Changes Induced by Naringenin-7-O-Glucoside in Triple-Negative Breast Cancer
Abstract
Introduction: Cancer is one of the most significant health challenges demanding the expansion of effectual therapeutic methods. Triple-negative breast cancer (TNBC) is a form of aggressive cancer with inadequate therapeutic options which lacks the expression of certain hormones.
Materials and methods: The present study investigates the potential of naringenin-7-O-glucoside, a flavanone glycoside extracted from Holarrhena antidysenterica as an anticancer agent against TNBC cell lines. In-vitro analysis to evaluate cytotoxicity, apoptotic-inducing properties and effect on gene expression was conducted.
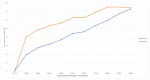
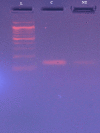
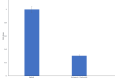
Results: MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay studied the IC-50 of naringenin-7-O-glucoside to be 233.56 µg/µL, revealing the dose-dependent cytotoxicity with minimal effect on Vero cells. Extensive DNA fragmentation confirmed the apoptotic property. Furthermore, a significant downregulation of the epidermal growth factor receptor (EGFR) was noted in treated cells when equated to the control specimen of the sample.
Conclusion: Therefore, naringenin-7-O-glucoside can be a potential targeted therapeutic agent.
Keywords: anticancer potential; egfr downregulation; naringenin-7-o-glucoside; selective cytotoxicity; triple-negative breast cancer.
Copyright © 2024, James et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Protective effects of naringenin-7-O-glucoside on doxorubicin-induced apoptosis in H9C2 cells.Eur J Pharmacol. 2008 Feb 26;581(1-2):47-53. doi: 10.1016/j.ejphar.2007.11.048. Epub 2007 Nov 29. Eur J Pharmacol. 2008. PMID: 18154951
-
Network Pharmacology-Based Identification of Key Mechanisms of Xihuang Pill in the Treatment of Triple-Negative Breast Cancer Stem Cells.Front Pharmacol. 2021 Oct 19;12:714628. doi: 10.3389/fphar.2021.714628. eCollection 2021. Front Pharmacol. 2021. PMID: 34737698 Free PMC article.
-
Chloroform Fraction of Methanolic Extract of Seeds of Annona muricata Induce S Phase Arrest and ROS Dependent Caspase Activated Mitochondria-Mediated Apoptosis in Triple-Negative Breast Cancer.Anticancer Agents Med Chem. 2021;21(10):1250-1265. doi: 10.2174/1871520620666200918101448. Anticancer Agents Med Chem. 2021. PMID: 32951586
-
Naringenin attenuates cell viability and migration of C6 glioblastoma cell line: a possible role of hedgehog signaling pathway.Mol Biol Rep. 2021 Sep;48(9):6413-6421. doi: 10.1007/s11033-021-06641-1. Epub 2021 Aug 24. Mol Biol Rep. 2021. PMID: 34427888
-
Cyanidin-3-o-glucoside directly binds to ERα36 and inhibits EGFR-positive triple-negative breast cancer.Oncotarget. 2016 Oct 18;7(42):68864-68882. doi: 10.18632/oncotarget.12025. Oncotarget. 2016. PMID: 27655695 Free PMC article.
Cited by
-
Morchella conica, Morchella esculenta and Morchella delicosa Induce Apoptosis in Breast and Colon Cancer Cell Lines via Pro-apoptotic and Anti-apoptotic Regulation.Chin J Integr Med. 2024 Sep 3. doi: 10.1007/s11655-024-3819-0. Online ahead of print. Chin J Integr Med. 2024. PMID: 39225882
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
