DNA damage response in brain tumors: A Society for Neuro-Oncology consensus review on mechanisms and translational efforts in neuro-oncology
- PMID: 38770568
- PMCID: PMC11300028
- DOI: 10.1093/neuonc/noae072
DNA damage response in brain tumors: A Society for Neuro-Oncology consensus review on mechanisms and translational efforts in neuro-oncology
Abstract
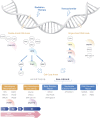
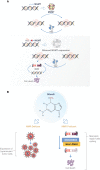
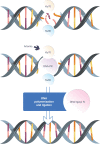
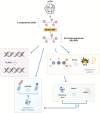
DNA damage response (DDR) mechanisms are critical to maintenance of overall genomic stability, and their dysfunction can contribute to oncogenesis. Significant advances in our understanding of DDR pathways have raised the possibility of developing therapies that exploit these processes. In this expert-driven consensus review, we examine mechanisms of response to DNA damage, progress in development of DDR inhibitors in IDH-wild-type glioblastoma and IDH-mutant gliomas, and other important considerations such as biomarker development, preclinical models, combination therapies, mechanisms of resistance and clinical trial design considerations.
Keywords: DDR inhibitors; DNA damage response; DNA repair; glioma; radiation therapy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
RR: Research support—Project Data Sphere; Research Support (to institution): Celgene Corporation, Puma Biotechnology, Inc., Eli Lilly and Company. Consulting—St. Lucia Consulting. ZJR: Listed as an inventor for intellectual property related to genetic testing for TERT and other alterations in brain tumors that are managed by Duke Office of Licensing and Ventures and has been licensed to Genetron Health; Honoraria—Oakstone Publishing and Eisai Pharmaceuticals. JdG: Advisory Board—Kintara Pharmaceuticals, Kazia, MundiPharma, Insightec, Monteris, Carthera, Samus, Sapience, DSP Pharma, Telix, Servier, Alpha Pharmaceuticals, CapitalOne; DSMB—Chimerix. Consulting: MundiPharma, Insightec, Carthera, Kintara, Deciphera, Kazia; Stock Ownership—Alaunos and Brii Biosciences (spouse). DHK: Consulting—GLG, Science Advisory Board—EmpNia Incorporated, Graegis Pharmaceuticals. NYRA is key opinion leader for Bruker Daltonics, receives support from Thermo Finnegan, EMD Serono, and iTeos Therapeutics. JNS: Research support—WayShine Biopharma and AstraZeneca. MM: Consulting: Telix, Kazia, Novocure, Zap, Xoft, Karyopharm, Sapience, Mevion; Board of Directors: Oncoceutics; Stock Ownership: Chimerix. PYW: Research Support—Astra Zeneca, Black Diamond, Bristol Meyers Squibb, Celgene, Chimerix, Eli Lily, Erasca, Genentech/ Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Servier, Vascular Biogenics, VBI Vaccines. Advisory Board/ Consultant -Astra Zeneca, Black Diamond, Celularity, Chimerix, Day One Bio, Genenta, Glaxo Smith Kline, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics, VBI Vaccines.
Figures
References
-
- Hegi ME, Diserens AC, Gorlia T, et al.. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. - PubMed
-
- Fishel R, Lee JB.. Mismatch Repair. In: Hanaoka F, Sugasawa K, eds. DNA Replication, Recombination, and Repair: Molecular Mechanisms and Pathology. Tokyo, Japan: Springer Japan; 2016:305-339. doi: 10.1007/978-4-431-55873-6_12 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

