Cyanovirin-N Binding to N-Acetyl-d-glucosamine Requires Carbohydrate-Binding Sites on Two Different Protomers
- PMID: 38770609
- PMCID: PMC11112747
- DOI: 10.1021/acs.biochem.4c00113
Cyanovirin-N Binding to N-Acetyl-d-glucosamine Requires Carbohydrate-Binding Sites on Two Different Protomers
Abstract
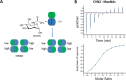
Cyanovirin-N (CV-N) binds high-mannose oligosaccharides on enveloped viruses with two carbohydrate-binding sites, one bearing high affinity and one low affinity to Manα(1-2)Man moieties. A tandem repeat of two CV-N molecules (CVN2) was tested for antiviral activity against human immunodeficiency virus type I (HIV-1) by using a domain-swapped dimer. CV-N was shown to bind N-acetylmannosamine (ManNAc) and N-acetyl-d-glucosamine (GlcNAc) when the carbohydrate-binding sites in CV-N were free to interact with these monosaccharides independently. CVN2 recognized ManNAc at a Kd of 1.4 μM and bound this sugar in solution, regardless of the lectin making amino acid side chain contacts on the targeted viral glycoproteins. An interdomain cross-contacting residue Glu41, which has been shown to be hydrogen bonding with dimannose, was substituted in the monomeric CV-N. The amide derivative of glucose, GlcNAc, achieved similar high affinity to the new variant CVN-E41T as high-mannose N-glycans, but binding to CVN2 in the nanomolar range with four binding sites involved or binding to the monomeric CVN-E41A. A stable dimer was engineered and expressed from the alanine-to-threonine-substituted monomer to confirm binding to GlcNAc. In summary, low-affinity binding was achieved by CVN2 to dimannosylated peptide or GlcNAc with two carbohydrate-binding sites of differing affinities, mimicking biological interactions with the respective N-linked glycans of interest and cross-linking of carbohydrates on human T cells for lymphocyte activation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Engineering recombinantly expressed lectin-based antiviral agents.Front Cell Infect Microbiol. 2022 Sep 23;12:990875. doi: 10.3389/fcimb.2022.990875. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36211961 Free PMC article.
-
Cyanovirin-N Binds Viral Envelope Proteins at the Low-Affinity Carbohydrate Binding Site without Direct Virus Neutralization Ability.Molecules. 2021 Jun 13;26(12):3621. doi: 10.3390/molecules26123621. Molecules. 2021. PMID: 34199200 Free PMC article.
-
The potent anti-HIV protein cyanovirin-N contains two novel carbohydrate binding sites that selectively bind to Man(8) D1D3 and Man(9) with nanomolar affinity: implications for binding to the HIV envelope protein gp120.J Am Chem Soc. 2001 May 2;123(17):3892-902. doi: 10.1021/ja004040e. J Am Chem Soc. 2001. PMID: 11457139
-
Cyanovirin-N: a sugar-binding antiviral protein with a new twist.Cell Mol Life Sci. 2003 Feb;60(2):277-87. doi: 10.1007/s000180300023. Cell Mol Life Sci. 2003. PMID: 12678493 Free PMC article. Review.
-
The highly specific carbohydrate-binding protein cyanovirin-N: structure, anti-HIV/Ebola activity and possibilities for therapy.Mini Rev Med Chem. 2005 Jan;5(1):21-31. doi: 10.2174/1389557053402783. Mini Rev Med Chem. 2005. PMID: 15638789 Review.
References
-
- Boyd R.; et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41 (7), 1521–30. 10.1128/AAC.41.7.1521. - DOI - PMC - PubMed
-
- Dey B.; Lerner D. L.; Lusso P.; Boyd M. R.; Elder J. H.; Berger E. A. Multiple antiviral activities of cyanovirin-N: blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J. Virol 2000, 74 (10), 4562–9. 10.1128/JVI.74.10.4562-4569.2000. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

