Strategies for increasing accrual in cancer clinical trials: What is the evidence?
- PMID: 38770644
- PMCID: PMC11106681
- DOI: 10.1002/cam4.7298
Strategies for increasing accrual in cancer clinical trials: What is the evidence?
Abstract
Introduction: Despite the importance of clinical trial participation among cancer patients, few participate-and even fewer patients from ethnic and racial minoritized groups. It is unclear whether suggested approaches to increase accrual are successful. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials that demonstrated clear increases in accrual. Notably, more stringent than other published reviews, only those studies with comparison data to measure a difference in accrual rates were included.
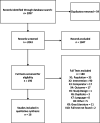
Methods: We searched PubMed/MEDLINE, Embase, CINAHL, and Web of Science for English-language articles published from January 1, 2012, to August 8, 2022. Studies were included if they were conducted in the United States, described single or multicomponent interventions, and provided data to measure accrual relative to baseline levels or that compared accrual rates with other interventions.
Results: Sixteen articles were included: six with interventions addressing patient barriers, two addressing provider barriers, seven describing institutional change, and one describing policy change. Key themes emerged, such as a focus on patient education, cultural competency, and building the capacity of clinics. Few studies provide comparative accrual data, making it difficult to identify with certainty any effective, evidence-based approaches for increasing accrual. Some patient- and system-level interventions studies showed modest increases in accrual primarily through pre-post measurement.
Conclusion: Despite an extensive body of literature about the barriers that impede cancer treatment trial accrual, along with numerous recommendations for how to overcome these barriers, results reveal surprisingly little evidence published in the last 10 years on interventions that increase accrual relative to baseline levels or compared with other interventions. As clinical trials are a primary vehicle through which we improve cancer care, it is critical that evidence-based approaches are used to inform all efforts to increase accrual. Strategies for increasing participation in cancer clinical trials must be developed and rigorously evaluated so that these strategies can be disseminated, participation in trials can increase and become more equitable, and trial results can become more generalizable.
Keywords: accrual; clinical trials.
© 2024 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
No authors report any conflicts of interest.
References
-
- National Comprehensive Cancer Network . Policy priority: ensure patient accessibility to guideline‐supported care. https://www.nccn.org/business‐policy/policy‐and‐advocacy‐program/policy‐...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials