3D Imaging Reveals Complex Microvascular Remodeling in the Right Ventricle in Pulmonary Hypertension
- PMID: 38770652
- PMCID: PMC11584150
- DOI: 10.1161/CIRCRESAHA.123.323546
3D Imaging Reveals Complex Microvascular Remodeling in the Right Ventricle in Pulmonary Hypertension
Abstract
Background: Pathogenic concepts of right ventricular (RV) failure in pulmonary arterial hypertension focus on a critical loss of microvasculature. However, the methods underpinning prior studies did not take into account the 3-dimensional (3D) aspects of cardiac tissue, making accurate quantification difficult. We applied deep-tissue imaging to the pressure-overloaded RV to uncover the 3D properties of the microvascular network and determine whether deficient microvascular adaptation contributes to RV failure.
Methods: Heart sections measuring 250-µm-thick were obtained from mice after pulmonary artery banding (PAB) or debanding PAB surgery and properties of the RV microvascular network were assessed using 3D imaging and quantification. Human heart tissues harvested at the time of transplantation from pulmonary arterial hypertension cases were compared with tissues from control cases with normal RV function.
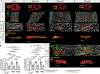
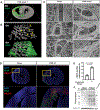
Results: Longitudinal 3D assessment of PAB mouse hearts uncovered complex microvascular remodeling characterized by tortuous, shorter, thicker, highly branched vessels, and overall preserved microvascular density. This remodeling process was reversible in debanding PAB mice in which the RV function recovers over time. The remodeled microvasculature tightly wrapped around the hypertrophied cardiomyocytes to maintain a stable contact surface to cardiomyocytes as an adaptation to RV pressure overload, even in end-stage RV failure. However, microvasculature-cardiomyocyte contact was impaired in areas with interstitial fibrosis where cardiomyocytes displayed signs of hypoxia. Similar to PAB animals, microvascular density in the RV was preserved in patients with end-stage pulmonary arterial hypertension, and microvascular architectural changes appeared to vary by etiology, with patients with pulmonary veno-occlusive disease displaying a lack of microvascular complexity with uniformly short segments.
Conclusions: 3D deep tissue imaging of the failing RV in PAB mice, pulmonary hypertension rats, and patients with pulmonary arterial hypertension reveals complex microvascular changes to preserve the microvascular density and maintain a stable microvascular-cardiomyocyte contact. Our studies provide a novel framework to understand microvascular adaptation in the pressure-overloaded RV that focuses on cell-cell interaction and goes beyond the concept of capillary rarefaction.
Keywords: fibrosis; heart failure; microvascular rarefaction; microvessels; pulmonary arterial hypertension.
Conflict of interest statement
Figures





References
-
- Haddad F, Doyle R, Murphy DJ, Hunt SA . Right Ventricular Function in Cardiovascular Disease, Part II: Pathophysiology, Clinical Importance, and Management of Right Ventricular Failure. Circulation. 2008;117:1717–1731. - PubMed
-
- Padang R, Chandrashekar N, Indrabhinduwat M, Scott CG, Luis SA, Chandrasekaran K, Michelena HI, Nkomo VT, Pislaru SV, Pellikka PA, Kane GC. Aetiology and outcomes of severe right ventricular dysfunction. Eur Heart J. 2020;41:1273–1282. - PubMed
-
- Zelt JGE, Chaudhary KR, Cadete VJ, Mielniczuk LM, Stewart DJ. Medical Therapy for Heart Failure Associated With Pulmonary Hypertension. Circ Res. 2019;124:1551–1567. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

