COVID-19 therapeutics
- PMID: 38771027
- PMCID: PMC11237566
- DOI: 10.1128/cmr.00119-23
COVID-19 therapeutics
Abstract
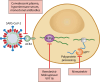
SUMMARYSince the emergence of COVID-19 in 2020, an unprecedented range of therapeutic options has been studied and deployed. Healthcare providers have multiple treatment approaches to choose from, but efficacy of those approaches often remains controversial or compromised by viral evolution. Uncertainties still persist regarding the best therapies for high-risk patients, and the drug pipeline is suffering fatigue and shortage of funding. In this article, we review the antiviral activity, mechanism of action, pharmacokinetics, and safety of COVID-19 antiviral therapies. Additionally, we summarize the evidence from randomized controlled trials on efficacy and safety of the various COVID-19 antivirals and discuss unmet needs which should be addressed.
Keywords: COVID-19; SARS-CoV-2; antiviral therapies; convalescent plasma; monoclonal antibodies; small-molecule antivirals.
Conflict of interest statement
D.F., M.F, and F.M. have no conflict of interest to declare. S.S. declares research funding from Ansun, F2G, and Zeteo and payment for consulting/data review activities from Adagio, Adamis, Celltrion, Karius, Pfizer, and Scynexis.
Figures
References
-
- Joyce RP, Hu VW, Wang J. 2022. The history, mechanism, and perspectives of nirmatrelvir (PF-07321332): an orally bioavailable main protease inhibitor used in combination with ritonavir to reduce COVID-19-related hospitalizations. Med Chem Res 31:1637–1646. doi: 10.1007/s00044-022-02951-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous